Method Development with ICP-MS/MS: Tools and Techniques to Ensure Accurate Results in Reaction Mode
Special Issues
Method setup and optimization steps are explored to illustrate how an ICP-MS/MS method can be defined and tested to ensure consistent performance. Users can benefit from improved interference removal performance without the complex method development inherent in the use of ion-molecule reaction chemistry
Since the launch of the first commercial triple-quadrupole ICP-MS (ICP-MS/MS) instrument in 2012, the technique has become well established in both research and academic institutes, as well as in industrial and commercial laboratories. Users in routine applications wish to benefit from the improved interference removal performance of ICP-MS/MS, without facing the more complex method development inherent in the use of ion-molecule reaction chemistry. In this article, we follow method setup and optimization steps to illustrate how an ICP-MS/MS method can be defined and tested to ensure consistent performance.
Single-quadrupole inductively coupled plasma–mass spectrometry (ICP-MS) provides performance that meets the needs of most inorganic (metals) analysis laboratories, and exceeds the method requirements for many. The technique offers a unique combination of fast multielement analysis, good elemental coverage, very low detection limits, and the ability to measure major and trace elements in a single acquisition. The development of collision-reaction cells (CRCs) operating in helium collision mode addressed many of the polyatomic interferences that previously hampered accurate analysis of several critical trace elements (1). However, helium mode cannot resolve interferences such as isobaric and doubly charged overlaps. Also, some combinations of analyte and interference require more effective attenuation of the overlap than can be achieved using collision mode and kinetic energy discrimination (KED). For these cases, ion-molecule reaction chemistry using a reactive cell gas may offer a better solution.
Reactive cell gas methods have been investigated since CRCs were first developed for single-quadrupole ICP-MS in the late 1990s (2). Early work presented the benefits of reaction chemistry for historically problematic analytes that suffer intense spectral overlap (such as selenium, arsenic, iron, calcium, and so on). However, these early applications were often based on the theoretical efficiency of the reaction chemistry to resolve one specific interference, and were illustrated using simple, consistent samples or synthetic solutions. Often, when these methods were applied to more complex and variable natural samples, it became apparent that the reaction processes could be adversely affected by other components in the sample (3,4). An unexpected matrix element or co-existing analyte could react with the cell gas, forming unwanted overlaps on the analyte ion (or product ion). Consequently, a reaction gas method developed for one sample matrix or interference might give erroneous results for a different sample type or combination of analytes.
The development of triple quadrupole ICP-MS (ICP-MS/MS) in 2012 offered a solution to the inaccurate results observed when using reactive cell gases on single quadrupole instruments (5). ICP-MS/MS has an additional quadrupole mass filter (Q1) before the CRC, in a tandem mass spectrometer (MS/MS) configuration. When Q1 is operated as a true mass filter (1 u mass window), it controls the ions passed to the cell, rejecting all nontarget masses. This means the reaction processes and product ions formed are predictable and consistent, and are unaffected by other analytes or matrix elements in the sample (6).
In addition to improving data quality, the control of reaction chemistry provided by ICP-MS/MS means that method settings can be applied consistently, even for variable samples. This makes reaction mode method setup easier than on a single quadrupole instrument or a configuration with a bandpass filter (mass window >1 u) before the cell. In this article, we illustrate the information and tools available for ICP-MS/MS to support development and performance testing of a new reaction mode method.
Reference Methods for Reactive Cell Gas Operation with ICP-MS/MS
Since the first commercial ICP-MS/MS instrument was released in 2012, many applications have been developed, optimized, and published. In addition to manufacturers' application notes (7), many hundreds of peer-reviewed journal articles detail method settings for both typical and unusual applications.
The ICP-MS/MS instrument's built-in methods can predefine the typical cell gas mode, analyte and product ion masses, and integration times for common analytes. Where an analyte does not have an established setting predefined in a preset method, the user can easily set up one or more mass pairs for the chosen reaction gas. The choice of reaction gas used to resolve a specific interference can be informed by the literature. Theoretical ion-molecule reaction thermodynamics show which reactions are favored (8,9), while ICP-MS/MS manufacturer's publications show experimental results for various product ion formation rates. Hydrogen (H2) oxygen (O2) and ammonia (NH3) are among the most widely-used reaction gases. For each gas, several common reaction transitions are defined in the literature, and have been confirmed experimentally (10).
With oxygen cell gas, for example, analytes may be measured on mass (Q2 is set to the same mass as Q1) or mass shifted as the oxide ion (Q2 is set to Q1 + 16 u) or dioxide ion (Q2 is set to Q1 + 32 u). These mass shift transitions, together with equivalent transitions for other reaction gases, are available as preset values in the method setup software, allowing easy selection of the preferred mass shift. Multiple mass shifts can be selected if the user wants to compare results for several product ions or cell gases, and custom mass shifts can be defined, if appropriate.
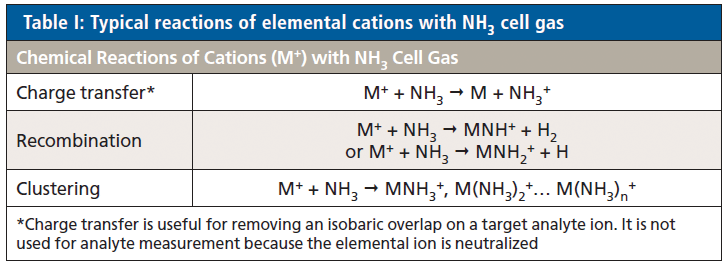
In the case of NH3 cell gas, reactions are usually fast and are often sequential, so multiple cluster ions can form from both the analyte ions and any other ions present in the cell. Typical reactions and product ions formed with NH3 cell gas are shown in Table I. MS/MS mode controls the precursor ions that can enter the cell and react, so product ions can only form from ions at the mass of the target analyte ion. However, optimized methods must still be verified to confirm that the chosen reaction product ion is free from overlap by product ions from other sample components present at the same mass as the analyte precursor ion.
Product Ion Scanning
Product ion scanning, which is unique to MS/MS, is a useful method development tool for unfamiliar sample types and unusual combinations of analyte interferences. This mode enables users to check and verify the best product ion(s) to use by identifying product ions that are free from overlap in a given sample type or matrix. This is particularly useful for complex and variable sample matrices, and for the large number of product ions produced when a highly reactive cell gas such as NH3 is used.
In a product ion scan, Q1 is set to the mass of the target analyte isotope, and Q2 is scanned across the mass range where useful product ions could appear. It is essential that Q1 is operated at 1 u resolution, so only ions at the target analyte isotope mass enter the cell. A product ion scan is acquired while aspirating a single-element standard of the target analyte, allowing product ions formed from the analyte isotope alone to be identified. A second product ion scan is then acquired while aspirating the unknown sample or synthetic matrix to identify product ions formed from other (nontarget) precursor ions at the target analyte mass. By comparing the spectra from the two scans, analyte product ions that are free from overlap by matrix product ions can easily be identified (11).
Experimental
Instrumentation and Reagents
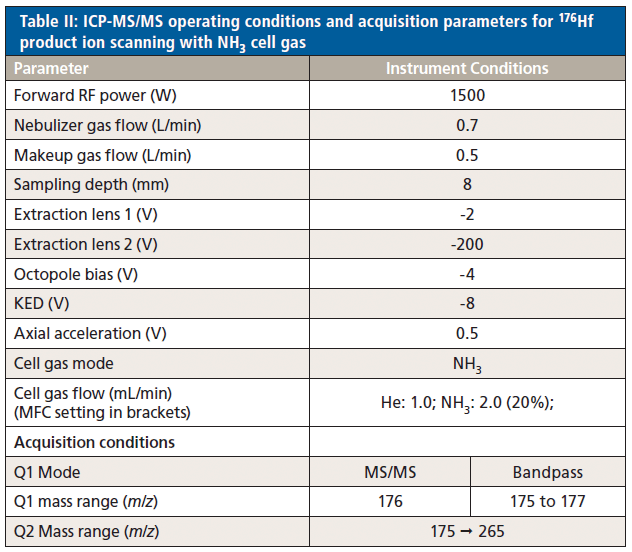
An Agilent 8900 ICP-MS/MS instrument (model #100, Advanced Applications configuration) was used for all measurements. The instrument was fitted with the standard glass concentric nebulizer, quartz spray chamber, quartz torch with 2.5 mm injector, and standard nickel sampling and skimmer cones. Samples were introduced via a peristaltic pump with 1.02-mm i.d. pump tubing. Operating conditions are shown in Table II.
The hafnium standard was prepared from a single element stock solution, and the rare earth matrix was prepared from a mixed 14-element stock standard (Agilent Technologies). Ultrapure water was prepared in the laboratory using a Milli-Q Element system, and nitric and hydrochloric acids used for sample stabilization were Ultrapure NORMATOM grade (VWR Chemicals).
Hf and mixed rare earth elements (REE) standards were prepared at 10 ppb and 1 ppm, respectively, in a final diluent of 2% HNO3 and 1% HCl. A spike solution of 10 ppb Hf in 1 ppm REE mix was also prepared.
Results and Discussion
Choosing Reaction Gas Modes to Resolve Known Interferences
Theoretical ion-molecule reaction rates can be used to identify potential approaches to resolve a given interference, based on differences in the reactions for the analyte and interfering ion. Figure 1 shows the reactions of all elemental ions with ammonia cell gas (10), displayed in a color-coded periodic table layout. To illustrate how this information can be used, the different reactions of Hg+ and Pb+ with NH3 are highlighted in the table. Mercury (a Type 3 element) reacts with NH3 cell gas via a charge-transfer reaction leading to the neutralization of the Hg+ ion. In contrast, Pb (a Type 1 element) does not react with NH3, so it can be measured on mass. The difference in reactions of Hg and Pb ions indicates that NH3 could be a suitable cell gas to resolve the 204Hg overlap on 204Pb, allowing accurate analysis of 204Pb on mass at m/z 204.
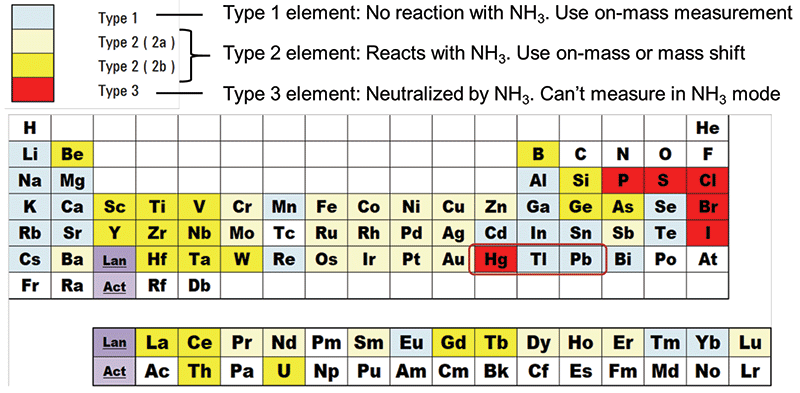
Figure 1: Reaction pathways and rates for all analyte ions with NH3 cell gas derived from reference 10. Hg and Pb are highlighted, showing how reaction data can be used to identify the potential to resolve the isobaric overlap from 204Hg on 204Pb.
In the case of hafnium analysis in a rare earth element matrix, the reaction data presented in Figure 1 suggests that NH3 cell gas might be able to resolve the Yb and Lu overlaps on Hf. The 178Hf isotope is the preferred isotope for quantitative analysis, but 176Hf is required for some applications such as Hf/Lu and Hf/Hf isotope geochronology. The 176Hf isotope could be considered the worst case in terms of interferences in sample matrices that contain REEs. As well as direct isobaric interferences from 176Yb and 176Lu,176Hf is also overlapped by oxide ions of 160Gd and 160Dy. Figure 2 shows that Hf (a Type 2b element) reacts with NH3 cell gas, whereas Yb (a Type 1 element) does not. Hafnium will therefore form Hf-NH3 product ions that cannot be overlapped by corresponding NH3 product ions formed from Yb.
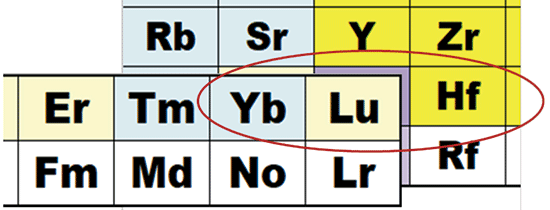
Figure 2: Reaction data indicates NH3 may be a suitable cell gas to resolve Yb and Lu overlaps on Hf (refer to Figure 1 for key to color-coding of NH3-reactions for each element).
The same principle does not necessarily apply to resolving the 176Lu isobaric overlap on 176Hf. Lu is a Type 2a element, so it does react with NH3, and may form product ions that can overlap the Hf-NH3 product ions. Also, ion-molecule reaction data does not give specific information about the potential for a given cell gas to resolve polyatomic overlaps, such as the 160Gd16O and 160Dy16O interferences on 176Hf. The solution to confirming the suitability of a given reaction gas to resolve such interferences is to verify the performance experimentally by using a product ion scan.
Identifying Interference-free Hf-NH3 Product Ions in REE Matrix Using Product Ion Scanning
For analytes, interferences, and sample types that are not defined in preset methods or available from reference publications, product ion scanning can identify the best product ion(s) for the application. Product ion spectra were acquired for a 10 ppb Hf standard and a 1 ppm 14-element REE mix. An overlay of the two product ion spectra covering the mass range from m/z 175 to 265 is shown in Figure 3. The spectrum comparison facilitates identification of product ions formed from m/z 176 precursor ions in the Hf standard but not the REE mix. The most intense interference-free 176Hf product ion identified was 176HfN(NH3)4+, which appears at m/z 258. This Hf product ion is free from any REE-based overlap-that is, no overlapping NH3 product ions are formed from Lu and GdO/DyO precursor ions present at m/z 176.
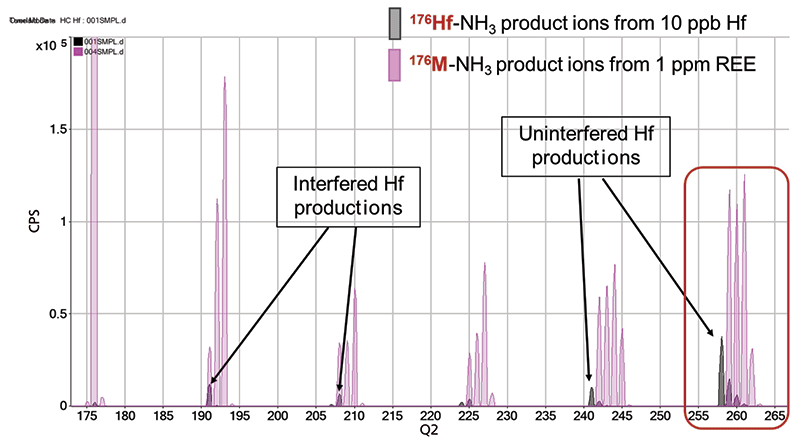
Figure 3: Overlaid spectra for NH3 product ions formed from m/z 176 in Hf standard (gray) and REE mix (pink), showing overlapped and interference-free Hf-NH3 product ions.
In this example, the REE-based overlaps being investigated were known in advance, so a synthetic standard solution could be prepared, ensuring the creation of the interfering REE-NH3 product ions. For complex sample matrices, which might give rise to unknown product ions, the product ion scan can be performed for a real sample matrix. In this way, potential overlaps from all sample components can be checked and accounted for during method development.
Controlling Product Ion Formation with MS/MS vs. Single Quadrupole or Bandpass
Product ion scanning-and the MS/MS methods developed using it - rely on Q1 operating at unit mass resolution (1 u mass window). The necessity for this is illustrated in Figure 4, where normal Agilent 8900 MS/MS operation is compared to deliberately "detuned" operation where Q1 has been set to pass a 3 u mass range. This simulates ICP-MS operation with a bandpass filter rather than a unit mass filter before the cell.
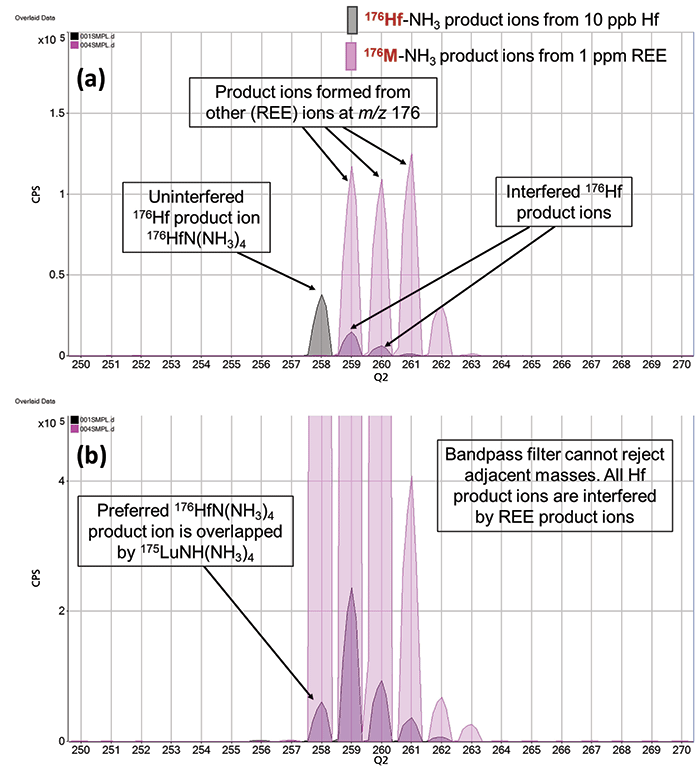
Figure 4: Detail showing NH3 product ions in the mass range around the preferred 176Hf product ion at m/z 258. In (a) MS-MS mode, 176HfN(NH3)4 is free from any REE product ion overlap. (b) In simulated 3 u bandpass mode, 175Lu is not excluded from the cell, so 175LuNH(NH3)4 is formed, overlapping 176HfN(NH3)4 at m/z 258.
The top spectrum in Figure 4 shows the mass range from m/z 250 to 270 extracted from the full mass product ion spectra in MS/MS mode shown in Figure 3. This includes the preferred, interference-free 176HfN(NH3)4+ product ion at m/z 258. The lower spectrum shows the same mass range for the same product ion scans for the 10 ppb Hf and 1 ppm REE solutions, acquired with 3 u bandpass mode. With a bandpass filter before the cell, adjacent precursor ions are not rejected, so they enter the cell and react. In this example, with Q1 passing a 3 u range of masses around the set mass, 175Lu can enter the cell when the precursor ion mass is set to m/z 176. 175Lu reacts with NH3 cell gas to form a product ion 175LuNH(NH3)4+, which appears at m/z 258, overlapping the preferred 176HfH(NH3)4+ product ion used for 176Hf analysis.
The example of interferences on Hf product ions in a REE matrix illustrates a general limitation of incomplete rejection of adjacent masses before the cell when using highly reactive cell gases. Any nontarget ions that enter the cell may react to form overlapping product ions, giving errors on the target analyte product ion mass.
Conclusions
Triple-quadrupole ICP-MS is often perceived as being more complicated and difficult to setup than single-quadrupole ICP-MS. However, the main benefit of the MS/MS tandem mass spectrometer configuration is the superior control it offers with reactive cell gas methods for interference removal. For these reaction gas methods, the ability of MS/MS to select the specific mass that is passed to the cell provides much greater control of the reaction chemistry. Rejecting nontarget, off-mass elements and isotopes using Q1 means these ions cannot enter the cell and affect the reaction processes. As a result, reaction gas methods on ICP-MS/MS are more consistent, more reliable, less matrix dependent, and therefore easier to setup than on single quadrupole ICP-MS.
References
(1) E. McCurdy and G. Woods, J. Anal. At. Spectrom. 19, 607–615 (2004).
(2) S.D. Tanner and V.I. Baranov, J. Am. Soc. Mass Spectrom. 10, 1083–1094 (1999).
(3) L.J. Moens, F.F. Vanhaecke, D.R. Bandura, V.I. Baranov, and S.D. Tanner, J. Anal. At. Spectrom. 16, 991–994 (2001).
(4) F. Vanhaecke, L. Balcaen, I. Deconinck, I. De Schrijver, C. Marisa Almeida, and L. Moens, J. Anal. At. Spectrom. 18, 1060–1065 (2003).
(5) S.D. Fernandez, N Sugishama, J.R. Encinar, and A. Sanz-Medel, Anal. Chem. 84, 5851–5857 (2012).
(6) N. Sugiyama, "The accurate measurement of selenium in twelve diverse reference materials using on-line isotope dilution with the 8800 Triple Quadrupole ICP-MS in MS/MS mode" (Agilent publication 5991-0259EN, 2012).
(7) Handbook of ICP-QQQ Applications using the Agilent 8800 and 8900, (Agilent publication 5991-2802EN, 2019).
(8) V.G. Anicich, Astrophys. J., Suppl. Ser. 84, 215–315 (1993).
(9) V.V. Lavrov, V. Blagojevic, G.K. Koyanagi, G. Orlova, and D. K. Bohme, J. Phys. Chem. A. 108, 26, 5610–5624 (2004).
(10) N. Sugiyama and K. Nakano, "Reaction data for 70 elements using O2, NH3 and H2 gases with the Agilent 8800 Triple Quadrupole ICP-MS" (Agilent publication 5991-4585EN, 2014).
(11) L. Balcaen, E. Bolea-Fernandez, M. Resano, and F. Vanhaecke, Anal. Chim. Acta809, 1–8 (2014).
Ed McCurdy and Glenn Woods are with Agilent Technologies in Stockport, United Kingdom. Naoki Sugiyama is with Agilent Technologies International, in Tokyo, Japan. Direct correspondence to: ed_mccurdy@agilent.com

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.