NIR Versus Mid-IR: How to Choose
Here the author compares near-infrared to mid-infrared as analytical tool in process management. he weighs the pros and cons of both spectral regions and suggests general applications for which one or the other is better suited.

Over the past twenty years, the use of the near infrared (NIR) region of the spectrum for a wide variety of the analytical procedures has grown precipitously. There are a number of instrument manufacturers providing a range of instruments from sophisticated costly spectrometers to recently introduced, lower cost, portable models. There are frequent seminars devoted to NIR use in different industrial areas. And because of the nature of NIR analytical data, a great deal of effort has gone into developing methods to sort out useful information from the generally weak and overlapping absorption material typical of NIR data.
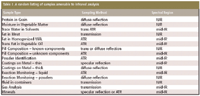
Table I: A random listing of samples amenable to infrared analysis
While NIR spectroscopy has found many broad applications in industrial process management, NIR advocates have sometimes tended to become overzealous in applying NIR to all industrial analyses, while in some cases, other procedures such as Raman and mid-IR spectroscopy could provide more useful analytical data more readily. After all, it is the mid-IR spectral region where most of the fundamental structural information is produced and when measurements in that region can be made on a particular class of samples, it may well be the method of choice. It is the purpose of the following sections to describe the overall characteristics of both spectral regions — the minuses as well as the plusses — to help the perspective user to select the spectral region best suited to his or her perspective uses.
NIR and Mid-IR Strengths and Weaknesses
NIR Strengths
1. Higher energy levels because radiation levels from black body emitters peak at shorter wavelengths.
2. High sensitivity photo conductive detectors function in the NIR but not in the mid-IR.
3. Water is reasonably transparent in this region, making it possible to use it as a solvent for some applications.
4. Perhaps most important, low-cost materials such as glass and quartz transmit NIR radiation and can be used as cell windows, focusing lenses, and optical fibers.
NIR Weaknesses
The major weakness of the NIR region is that the absorption bands occurring there are the overtones of the fundamental bands residing in the mid-IR region. As a result, they are relatively weak and not clearly delineated. This makes quantitative calculations complex and calibration procedures quite laborious and not transferable from one instrument to another.
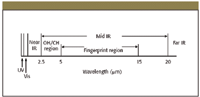
Figure 1: A portion of the electromagnetic spectrum showing the relationship between NIR and mid-IR.
NIR Applications
In spite of the above limitations, NIR equipment is becoming widely used in such applications as diffuse reflection measurements on foods, monitoring pharmaceutical processes, in-situ biomedical applications, to name a few.
Mid-IR Strengths
1. Organic functional groups have characteristic and well delineated absorption bands in this spectral region.
2. Because molecules differ from each other by having different combinations of functional groups, their mid-IR spectra can be used to identify them and characterize their structure.
3. Mid-IR spectra of mixtures are additive. Absorption bands associated with individual components in a mixture are frequently isolated from other bands and can be used to quantify the individual components by the strength of their absorption.
4. Calibration data in the mid-IR is much more generic than that in the NIR and thus is more readily transferable from instrument to instrument.
Mid-IR Weaknesses
1. As a result of the aforementioned black-body radiation curve, available energy in the mid-IR decreases substantially with wavelength.
2. Mid-IR transmitting materials are more expensive and in some cases less chemically resistant. Mid-IR optical fibers tend to be very expensive and quite difficult to manipulate.
3. Most materials are strongly absorbing in the mid-IR and therefore cells with extremely short effective pathlengths – in the neighborhood of 10–100 μm – must be used.
Mid-IR Applications: Mid-IR is best suited for materials identification for example, "white powders," incoming raw material verification, etc.; process monitoring through the increase or decrease of a functional absorption band, i.e., the carbonyl 5.7 μm band; determination of a specific component in a mixture such as concentration of trans fatty acid in vegetable oil, alcohol levels in beverages; gas analysis in general.
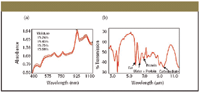
Figure 2: Typical near and midspectra of food stuff.
Typical Uses of Infrared Analysis by Industry
Infrared in the Pharmaceutical Industry: With the recent directives from FDA that pharmaceutical processes should be monitored stage by stage (process analytical technology [PAT]), infrared procedures, both NIR and mid-IR have found expanding applications. NIR, because of its ability to penetrate deeply into materials, is especially useful for QC measurements on pills and powders while mid-IR, through the use of attenuated total reflection (ATR), works best in following reactions in liquid process streams and reaction vessels.
Infrared in Food Processing: While the food industry has lagged the pharmaceutical industry in applying analysis in the food processing plant, the availability of portable, environmentally sound instruments both NIR and mid-IR plus new government regulations is creating new uses for IR in general for better quality control. Among the NIR applications are protein levels in grain by diffuse reflectance, online moisture levels, fat content in meats. Developing mid-IR uses are trans fatty acid in vegetable oils, alcohol, carbonation, and sugar in beverages. Many other uses for both spectral regions will become apparent as the industry appreciates the applicability and ruggedness of the new portable instruments.
Infrared in the Chemical Industry: Infrared along with chromatography and mass spectrometry have long been the analytical mainstays of chemical processing though usually carried out in the laboratorys. IR, both near and mid is the best suited analytical method to move analysis out of the laboratory into the plant because, unlike other techniques, infrared is nondestructive. With the new linear variable filter array spectrometers, optical filters, rugged and sensitive non cooled detectors among other developments, infrared devices of all wavelengths (including UV and vis!) are being used more and more both for in-line and at-line monitoring.
Environmental Monitoring: Mid-IR techniques have long been widely used for such uses as measuring petroleum hydrocarbons and fats, oil, and grease in wastewater and soil because of its specificity and sensitivity.
Nearly all nondiatomic gases have characteristic absorption bands in the seven to fourteen micrometer region of the mid-IR spectrum. Mid-IR gas analyzers with long path gas cells are the basic tool for toxic vapor monitoring in ambient air.
NIR and Mid-IR Together Form a Powerful Team
The purpose of this discussion is not to prove that mid-IR is more useful than NIR or visa versa but to demonstrate that infrared analysis in general is an increasingly powerful procedure that is finding increasingly wide usefulness both because of its fundamental characteristics and as a result of newly developed instrument components.
Table I is a completely random listing of sample categories that have proved amenable to various types of infrared sampling approaches. Those engineers charged with the responsibilities of applying analytical procedures to their particular processes should do so with open minds: not determined to make only NIR or only mid-IR work, but to study the problem objectively to determine how to go about solving it. All infrared procedures will play key roles in the future in PAT and other at-line and in-line monitoring applications.
Paul Wilks is president of Wilks Enterprise, Inc. (South Norwalk, Connecticut).

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Real-Time Battery Health Tracking Using Fiber-Optic Sensors
April 9th 2025A new study by researchers from Palo Alto Research Center (PARC, a Xerox Company) and LG Chem Power presents a novel method for real-time battery monitoring using embedded fiber-optic sensors. This approach enhances state-of-charge (SOC) and state-of-health (SOH) estimations, potentially improving the efficiency and lifespan of lithium-ion batteries in electric vehicles (xEVs).