Optimized ICP-MS Analysis of Elemental Impurities in Semiconductor-Grade Hydrochloric Acid
Special Issues
A closer look at the use of a cell-based ICP-MS approach that utilizes ion–molecule chemistry to reduce many of the traditional spectral interferences seen in the analysis of high-purity hydrochloric acid used in manufacturing integrated circuits and semiconductor devices
This article focuses on the use of a cell-based inductively coupled plasma–mass spectrometry (ICP-MS) approach that utilizes ion–molecule chemistry to reduce many of the traditional spectral interferences seen in the analysis of high-purity hydrochloric acid used in manufacturing integrated circuits and semiconductor devices. In addition, a solid-state radio frequency (RF) generator allows the use of lower powers to reduce formation of argon-based polyatomic interferences while still maintaining strong ionization conditions. The combination of controlled gas-phase reaction chemistry with the unique RF generator results in the elimination or significant reduction of many of the problematic chloride-, water-, and argon-based polyatomic spectral interferences, allowing the determination of a suite of 43 ultratrace elements in semiconductor-grade hydrochloric acid by following the validated analytical protocols described in the Semiconductor Equipment Manufacturers International (SEMI) Tier C guidelines.
The semiconductor industry provides one of the most demanding application areas for inductively coupled plasma–mass spectrometry (ICP-MS) with regard to the detection capability requirements. Consumer demand for smaller electronic devices and more compact integrated circuits has resulted not only in the need for lower trace metal contamination levels on the surface of silicon wafers, but also in the high-purity chemicals and gases used in various stages of the semiconductor manufacturing process. To reduce costs and increase yield, chip manufacturers are making larger diameter wafers with ever smaller chip components. This trend, which is being driven by initiatives like the International Technology Roadmap for Semiconductors (ITRS) (1), is setting the course for the next generation of semiconductor devices and has resulted in requirements for lower and lower trace-element contamination levels in all semiconductor-related materials. Whereas 20 years ago the Semiconductor Equipment Manufacturers International (SEMI) organization deemed that 10-ppb (µg/L) purity levels were adequate for many of the process chemicals, today Tier C levels of 100 ppt (ng/L) are typical-and for some of the more critical chemicals, 10-ppt (ng/L) levels are required (2).
Tier C refers to third-generation guidelines generated by the SEMI North American Process Chemicals Committee and published in its Book of Semiconductor Standards (BOSS) (3). The guidelines are intended to describe the quality required to produce integrated circuits whose critical dimensions lie in the range of 0.09–0.2 µm. Hydrochloric acid (HCl) is currently in the BOSS at the Tier C guideline level, meaning that the maximum contaminant level of 18 elemental impurities should each be less than 100 ppt (ng/L) in the process chemical. On completion and approval of the validated analytical method described in the guideline, it will meet the SEMI Grade 4 Standard (4).
Discussion
Traditionally, ICP-MS has been the technique of choice for ultratrace-element determinations in many of the high-purity chemicals used in the semiconductor and electronics industries. However, to get to the next required purity level of 10 ppt (ng/L) and beyond, conventional ICP-MS has shown that it does not have the detection capability for the full suite of trace metals-even with the use of spectral background reduction techniques like cool or cold plasma conditions and collision-cell technology. To achieve the next required analytical levels, a cell-based ICP-MS approach capable of using pure, highly reactive gases to produce targeted, controlled reactions to eliminate argon-, matrix-, and solvent-based spectral interferences is required. When combined with optimized plasma conditions, detection limits can be dramatically lowered for many of the semiconductor elements.
It is well-recognized that the ability to control ion-molecule chemistry in cell-based ICP-MS is essential when using the highly reactive gases required for maximum removal of atomic and polyatomic spectral interferences to meet the SEMI Tier C levels and beyond (5,6). For example, consider the analysis of hydrochloric acid, which requires the removal of the chloride-based interferences such as oxygen-chloride (16O35Cl+) on vanadium at m/z 51 and argon-chloride (40Ar35Cl+) on arsenic at mass 75. In addition, other prominent interferences include argon-oxide (40Ar16O+) on iron at mass 56 and chlorine-hydroxide (35Cl16OH+) on chromium at m/z 52. Pure ammonia is the most effective gas for removing 16O35Cl+, 40Ar16O+, and 35Cl16OH+ while allowing for low-parts-per-trillion (nanograms-per-liter) determinations of vanadium, iron, and chromium (7), limited only by levels of contamination. Oxygen has proven most effective for the determination of arsenic in hydrochloric acid by reacting with 75As+ to form 75As16O+ at m/z 91, where it is measured interference-free, away from the 35Cl40Ar+, which does not react.
In addition, the use of cold plasma conditions has been very effective at reducing the number of argon ions formed in the plasma, which minimizes the formation of argon-based species such as 40Ar+ and 38ArH+ and facilitates the determination of elements like 40Ca+ and 39K+ (8). Even though cold plasma has been used for many years, it has several limitations, including the ability to deal with real-world matrices such as concentrated mineral acids, susceptibility to matrix suppression effects, and the inability to switch rapidly between cold and normal plasma conditions during a multielement analysis.
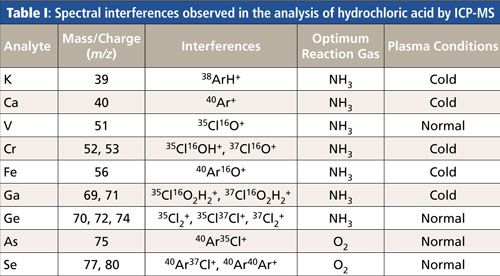
However, advances in solid-state free-running radio frequency (RF) generators have enhanced the capability of ICP-MS to analyze real-world samples using cold and normal plasma conditions (9). The combination of pure, highly reactive gases in a controlled reaction environment with cold plasma conditions provides the most efficient reduction of polyatomic interferences, allowing ultratrace levels to be accurately measured. Table I shows chloride-, water-, and argon-based spectral interferences, which can potentially interfere with a suite of analyte masses during the analysis of hydrochloric acid, together with the optimum reaction gas and plasma conditions.
Investigation
The goal of this investigation was to meet or exceed the SEMI Tier C guideline levels for the analysis of semiconductor-grade hydrochloric acid. This guideline states that spike additions made at 50% of the proposed specification level (100 ppt in 37% HCl) must have recoveries within 75–125% of the expected value. To evaluate the long-term stability, a 10-h run was performed monitoring a solution of 20% HCl spiked with 50 ppt of all elements.
Experimental
Samples and Sample Preparation
In semiconductor plants, high-purity HCl (35–38%) is most commonly used as an etchant in the chip fabrication process. One of the main requirements is that it must contain extremely low levels of elemental impurities so the etching process does not deposit elemental contaminants on the surface of the wafer or chip. To analyze high-purity HCl by ICP-MS, it is typically diluted twofold with ultrapure deionized water before aspiration into the instrument. To eliminate potential contamination from dilution, ultrapure 20% HCl (Tamapure-AA-10, Moses Lake Industries) was used in this work and analyzed directly without dilution. Calibration standards (10, 20, and 40 ng/L) were made from 10-mg/L multielement stock solutions via serial dilution, with the final standards being prepared in 20% HCl. Spikes of 10 and 25 ppt in the 20% HCl were used to evaluate recovery. Although the 25-ppt spike meets the SEMI Tier C guidelines, the 10-ppt spike was also measured to evaluate the analytical methodology with an eye toward future SEMI requirements.
Instrumental Conditions
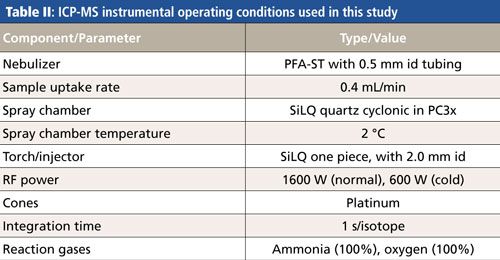
All analyses were performed on a NexION 2000 S ICP-MS system (10) (PerkinElmer, Inc.) using the instrumental parameters shown in Table II.
Analyses were accomplished with a combination of reaction (with NH3 and O2) and standard modes (no cell gas), using either normal plasma (1600 W) or cold plasma (600 W) conditions for the 43-analyte multielement method. To maximize the effectiveness of reaction mode, 100% ammonia and 100% oxygen were used in two different ways: Ammonia effectively removed interferences at the analytical mass, and oxygen was used to shift the analyte to a new analytical mass. The latter approach was found to be most effective for the measurement of arsenic and selenium. Table sI (available online) displays the method parameters for all 43 analytes.
Results
Interference Reduction Strategies Using Reaction Mode
It is important to emphasize that the effects of interferences were removed in reaction mode in two different ways:
- removing the interference at the analytical mass, and
- shifting the analyte to a new mass away from the interference.
An example of removing the interference at the analytical mass is the removal of the 35Cl16O+ on V+ at mass 51. Although ammonia reacts rapidly with 35Cl16O+ to remove it, the reaction is driven to completion by using 100% ammonia in the cell. In addition, because the reaction cell incorporates a quadrupole, the RF-only parameter (RPq) acts as a low mass filter to control the chemistry and prevent the formation of new interferences, which is critical when using highly reactive gases. This is particularly important in removing the 35Cl16O+ interference since one of the intermediate products is 35Cl+, which reacts with NH3 gas to form 35Cl14NH2+ at mass 51. However, because the RPq parameter serves as a low mass filter, it can be set to make 35Cl+ unstable in the cell, which prevents the formation of 35Cl14NH2+ and allows 51V+ to be measured free of interferences.
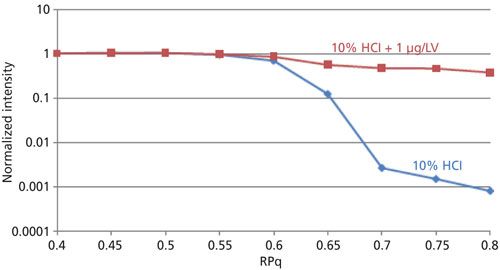
This functionality is demonstrated in Figure 1, which shows the optimization of the RPq parameter for 1 μg/L V in 10% HCl. As the RPq parameter increases to 0.7, the background decreases sharply, which represents where 35Cl+ is no longer stable in the cell (blue plot). As a result, 35Cl+ is ejected from the cell, meaning that the reaction to 35Cl14NH2+can no longer occur, resulting in interference-free analysis of V+ at mass 51 (red).
Figure 1: Signal intensities at mass 51 as a function of RPq for 10% HCl (blue) and 10% HCl + 1 μg/L V (red).
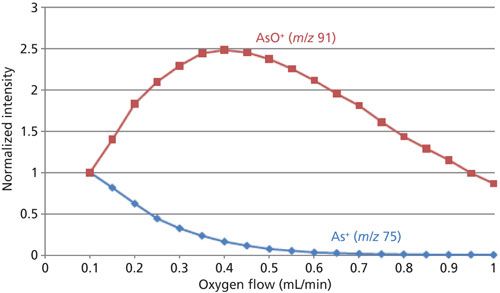
The second method of dealing with interferences is using reaction chemistry in mass-shift mode where the analyte reacts with the reaction gas moving it to a new analytical mass. An example of this approach is the analysis of arsenic (As), which reacts rapidly with oxygen to form 75As16O+ at mass 91, away from the 40Ar35Cl+ interference at mass 75. Because 40Ar35Cl+ does not react with oxygen (O2), 75As16O+ is measured free of interferences. Figure 2 shows the conversion of 75As+ to 75As16O+ as a function of oxygen flow. As the O2 flow increases, the signal for 75As+ decreases (blue plot), while the signal for 75As16O+ increases (red plot), demonstrating complete conversion.
Figure 2: Conversion of 75As+ to 75As16O+ as a function of oxygen flow in 1% HNO3.
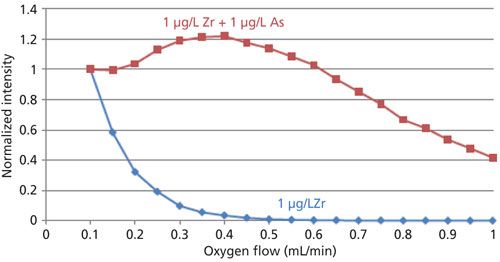
One of the concerns of measuring 75As16O+ at mass 91 is that the presence of zirconium (91Zr+) in a sample may produce a false positive reading. However, because 91Zr+ reacts rapidly with oxygen (rate constant ≈ 10-10), it is removed from m/z 91 as 75As16O+ forms, allowing for interference-free measurement of 75As16O+ even if Zr is present at the same concentration as As, as shown in Figure 3. In this figure, mass 91 is monitored as a function of oxygen flow for a 1-μg/L Zr standard (blue) and a mixed standard containing 1 μg/L Zr + 1 μg/L As.
Figure 3: Signals at mass 91 as a function of oxygen flow rate for 1 μg/L Zr (blue) and 1 μg/L Zr + 1 μg/L As (red).
Detection Capability Performance
To assess the effectiveness of interference removal, method detection limits (MDLs) and background equivalent concentrations (BECs) were determined in 20% HCl using a 1-s integration time. The MDLs (analyte signal and background noise) were calculated by multiplying the standard deviation of the blank (10 analyses) by three, and the BECs (analyte signal and background signal) were determined by measuring the background concentrations of the blank at each analyte mass. A spike recovery test was carried out based on SEMI Tier C guidelines, but using a lower spike level of 10 ppt (ng/L), Table sII (available online) shows both the MDLs and BECs for all elements, as well as the 10-ppt (ng/L) spike recoveries. The majority of MDLs and BECs are less than 1 ng/L, demonstrating the effectiveness of using both controlled reaction chemistry and cold plasma conditions. All spike recoveries fall within 10% of the true values, showing the accuracy of low-level measurements.
Calibration
Calibration curves were established with 10-, 20-, and 40-ng/L (ppt) standards using the method of standard additions. All curves had regression values of >0.999, demonstrating both the linearity of the analysis and the ability to accurately measure at low concentrations. Figure s1 (available online) shows three calibration curves, which highlight the effectiveness of the interference reduction: calibration curves for 40Ca+ and 56Fe+ were acquired using ammonia as the reaction gas and cold plasma conditions, demonstrating the ability to remove significant plasma-based interferences (40Ar+, 40Ar16O+). 51V+ was acquired with ammonia and normal plasma conditions, exemplifying how effectively a significant chloride interference (35Cl16O+) can be removed. The accuracy of low-level measurements was then verified by measuring the recoveries of 10-ng/L (ppt) spiked additions in 20% HCl, which are all within 10% of the true value.
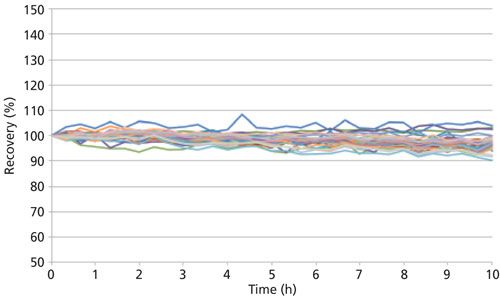
With the ability to remove interferences and quantitative accuracy established, the stability of the methodology was evaluated by measuring a 50-ng/L (ppt) spike in 20% HCl over 10 h of continuous aspiration. The results shown in Figure 4 demonstrate the stability for all 43 elements when using a mixed-mode method consisting of standard (no cell gas) and reaction (NH3/O2) modes with both normal and cold plasma conditions. These data show that for every element the drift of the signal is less than 10% from the initial reading, while the RSDs over the 10 h are less than 3% for all elements.
Figure 4: A 10-h stability plot of 50 ng/L analyte spikes in 20% HCl, with continuous aspiration.
Supplementary Information Online
Tables sI and sII as well as Figure s1 are available online in a supplementary information section. Please visit www.spectroscopyonline.com/supplementary-information-optimized-icp-ms-analysis-elemental-impurities-semiconductor-grade-hydroch to view that content.
Conclusion
This study has demonstrated the ability of the methodology used in this investigation to perform the elemental analysis of 20% HCl at SEMI Tier C guideline levels and beyond, without the need for dilution. The combination of fast switching between hot and cold plasma together with reaction cell technology using 100% ammonia and oxygen led to the elimination of spectral interferences on many of these elements. This capability allows for sub-part-per-trillion detection limits, excellent spike recoveries and accurate quantitation at the 100-ppt Tier C guideline level while offering the kind of long-term stability required by a high-throughput semiconductor production laboratory. In addition, the data show that quantitation at the SEMI Tier D guideline level (proposed Grade 5) of 10 ppt can be achieved.
References
- International Technology Roadmap for Semiconductors (ITRS 2.0), Semiconductor Industry Association (2015), http://www.semiconductors.org/main/2015_international_technology_roadmap_for_semiconductors_itrs/.
- Semiconductor Equipment and Materials International (SEMI) (Organization, Milpitas, California, http://www.semi.org/en/).
- SEMI Book of Standards (BOSS): Process Chemicals (Updated periodically, http://ams.semi.org/ebusiness/standards/semistandard.aspx?volumeid=13).
- SEMI C27-0708 - Specifications and Guidelines for Hydrochloric Acid, http://ams.semi.org/ebusiness/standards/SEMIStandardDetail.aspx?ProductID=211&DownloadID=30.
- S.D. Tanner and V.I. Baranov, At. Spectrosc. 20(2), 45–52 (1999).
- K. Kawabata Y. Kishi, and R. Thomas, Anal. Chem. 75(9), 423A (2003).
- Y. Kishi and K. Kawabata, At. Spectrosc.23(5), 165–169 (2002).
- S.D. Tanner et al., At. Spectrosc. 16(1), 16–18 (1995).
- T.S. Cheung, C. Wong, and H.R. Badiei, Technical Note, PerkinElmer Inc., Woodbridge, Ontario, Canada.
- NexION 2000 ICP-MS System: Product Description, http://www.perkinelmer.com/lab-solutions/resources/docs/BRO-NexION-2000-ICP-MS-012730_01.pdf.
Ken Neubauer and Ewa Pruszkowski are with PerkinElmer, Inc., in Shelton, Connecticut. Direct correspondence to: kenneth.neubauer@perkinelmer.com

Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.
Best of the Week: Hyperspectral Imaging, ICP-MS Analysis of Geological Samples, Product Roundup
October 18th 2024Top articles published this week include an article about hyperspectral imaging in human skin research, a peer-reviewed article about analyzing geological samples using atomic spectroscopy techniques, and an equipment roundup piece about the latest products in the industry.