Polymorph Characterization Using the Thermo Scientific Nicolet iS50 Raman Module
Solid dose drugs comprise a considerable segment of the pharmaceutical market.
Solid dose drugs comprise a considerable segment of the pharmaceutical market. The solid dose form offers long shelf life, convenient drug delivery, and can be packaged into capsules, tablets, gel caps, and patches. One concern is polymorphism, the ability of a molecule to exist in more than one stable or meta-stable crystalline state. Distinct crystalline forms commonly exhibit differences in pharmacologically relevant properties of an active pharmaceutical ingredient (API). The affected properties may include bioavailability, solubility, absorption rate, stability or manufacturability. Since polymorphism can have profound effects on an API's pharmacodynamics and manufacturing process, it becomes critical to characterize and understand polymorphic behavior.
The information-rich content of Raman spectra and the availability of reliable commercial Raman instruments have given rise to the use of Raman spectroscopy in many aspects of drug development (1–3). Raman spectroscopy has proven to be a valuable analytical method for differentiating between different polymorphs, salts, co-crystals, solvates, and hydrates of drug candidates.
In this application note, we describe using FT-Raman to characterize different polymorphs of a model compound, 5-Methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile (ROY) (4–6).This compound was kindly provided by Professor Lian Yu, of the University of Wisconsin, School of Pharmacy, Division of Pharmaceutical Sciences. This unique compound exhibits different colors for different polymorphs, and we describe here the analysis of three ROY polymorphs (red, orange, and yellow) seen in Figure 1.
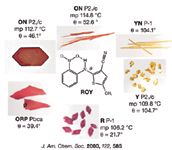
Figure 1: Pictures of various polymorphs found for 5-Methyl-2-[(2-nitrophenyl)amino]-3-thiophenecarbonitrile (ROY).
Experimental Methods
The spectra described in this paper were acquired with the Thermo Scientific™ Nicolet™ iS™50 FT-IR spectrometer and the iS50 Raman module. The iS50 Raman module mounts in the sample compartment of the Nicolet iS50 spectrometer and is equipped with a 1064 nm near-infrared laser. ROY samples were mounted in a mini-well plate that was placed on the integrated, automated X, Y, Z stage for alignment. Figure 2 shows a combined image of the wells containing the three polymorphs.

Figure 2: This composite image from the iS50 Raman module shows the three ROY polymorphs in three 1 mm diameter wells in a mini-plate. Starting from the left; red, yellow, and orange polymorphs.
The spectra from the three samples exhibit a number of significant spectral differences as seen in Figure 3. One clear difference is the position of the nitrile peak near 2200 cm-1 that has been expanded in Figure 4. This peak shift appears to correlate with the different angle observed for the nitrile functional group in the different crystal forms.
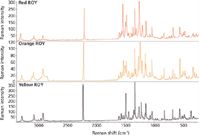
Figure 3: Full Raman spectra from three polymorphs of ROY.
Prolonged exposure of the orange polymorph to the excitation laser produced an interesting effect. Spectra collected over time at high laser power showed an increased presence of a second nitrile peak that closely matched the peak from the yellow polymorph. Care must be taken to prevent polymorphic changes due to the laser. Figure 5 shows the resulting mixture spectra on the bottom with the three pure polymorphs displayed on top. Curve fitting using the locations of the peaks from the independent polymorphs gave the excellent fit shown in Figure 6, demonstrating that Raman spectra can be used to determine the relative amount of different polymorphs present in a sample.
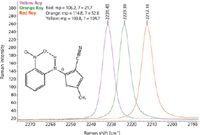
Figure 4: Expansion of the nitrile peak showing shift for three different ROY polymorphs.
Raman spectroscopy provides pharmaceutical companies with a versatile tool for identifying the proper polymorph (or polymorph blend) during drug discovery. This allows optimization of the production process to yield the desired product and speeds scale-up work into manufacturing. Many classes of laboratory — from research to forensics — can benefit from the additional information FT-Raman spectroscopy offers in understanding polymorphism and crystallinity.
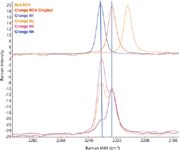
Figure 5: A comparison of the spectra from the pure materials (top) with spectra from samples containing a mixture of two polymorphs.
Conclusion
Analyzing the polymorphs and salt forms of an API has become a significant step in the drug development process, due to their affect on drug performance and stability which can drastically change critical characteristics in vivo. Further, identifying and comparing the properties of different polymorphs, salts, solvates, complexes, and co-crystals during drug discovery is vital for building intellectual property and creating a competitive advantage.
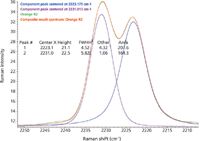
Figure 6: Results of curve fitting that provide a good estimate of relative concentrations from peak heights or areas for each polymorph.
FT-Raman units such as shown in Figure 7 can be extremely valuable for characterizing these polymorphs and mixtures during drug development as well as providing a tool for identifying counterfeit drugs or patent infringement. FT-Raman analysis is fast, requires little to no sample preparation and can be used to characterize bulk or single crystal samples. Combined with other tools, such as DSC and X-ray crystallography, FT-Raman can speed laboratory operations and simplify manufacturing QA/QC, even with samples contained in sealed bottles.

Figure 7: The Thermo Scientific Nicolet iS50 spectrometer with the iS50 Raman module in the sample compartment with a well plate designed for analyzing solid dose tablets.
References
(1) S. Lowry, D. Dalrymple, A. Song, V. Rosso, C. Pommier, and J. Venit, "Integrating a Raman Microscope into the Workflow of a High-Throughput Crystallization Laboratory" J. Assoc. Lab. Autom. 11(2), 75–84 (2006).
(2) D. Igo and P. Chen, "Vibrational Spectroscopy of Solid-State Forms – Application and Examples" Applications of Vibrational Spectroscopy in Pharmaceutical Research and Development; D. Pivonka, J. Chalmers, P. Griffiths, Eds. (John Wiley & Sons: New York, 2007), pp. 293–308.
(3) D. Bugay, J. Henck, M. Longmire, and F. Thorley, "Raman Analysis of Pharmaceuticals," Applications of Vibrational Spectroscopy in Pharmaceutical Research and Development; D. Pivonka, J. Chalmers, and P. Griffiths, Eds. (John Wiley & Sons: New York, 2007) pp. 239–262.
(4) L. Yu, "Red, Orange and Yellow Polymorphs of a Conformationally Flexible Molecule: Single-Crystal Spectroscopy, Optical Crystallography and Computational Chemistry," J. Phys. Chem. A 106, 544–550 (2002).
(5) S. Chen, I. A. Guzei, and L. Yu, "New Polymorphs of ROY and New Record for Coexisting Polymorphs of Solved Structures," J. Am. Chem. Soc. 127, 9881–9885 (2005).
(6) L. Yu, "Polymorphism in Molecular Solids: An Extraordinary System of Red, Orange, and Yellow Crystals," Acc. Chem. Res. 43, 1257–1266 (2010).
Thermo Fisher Scientific, Molecular Spectroscopy
5225 Verona Rd., Madison, WI 53711
tel. (608) 276-6100, fax (608) 276-5046
