Portable Spatially Offset Raman Spectroscopy for Rapid Hazardous Materials Detection Within Sealed Containers
Special Issues
Portable spatially offset Raman spectroscopy (SORS) enables rapid identification of materials concealed by a wide variety of nonmetallic sealed containers. This technique has been successfully used by the military, first responders, and customs and law enforcement operators in the detection of explosives, chemical agents, precursors and hazardous narcotics, thus enabling the acquisition of better information without disturbing the materials in question.
Raman spectroscopy is an optical spectroscopic method that enables characteristic identification of chemical substances. Spatially offset Raman spectroscopy (SORS), a novel variant of Raman spectroscopy, enables new capabilities in the rapid identification of materials concealed by a wide variety of nonmetallic sealed containers, such as colored and opaque plastics, paper, cardstock, sacks, fabric, and glass, which are often challenging for conventional backscattered Raman spectroscopy. Handheld spectroscopy based on SORS has been developed for detection of several materials and barrier types, including explosives, chemical agents, precursors, and hazardous narcotics (such as fentanyl). SORS has the potential to improve the safety, efficiency, and critical decision-making in incident management and response. The operator is able to obtain a positive identification of a potentially hazardous material without opening or disturbing the container-to gain access to take a sample-thus improving safety. The technique is fast and simple, therefore protective suit and breathing gear time is used more efficiently when operating in a "hot zone".
There is a continuing evolution of new threats and chemical hazards throughout the world. Therefore, a method that can positively identify these threats, while being user friendly, highly specific, fast, and safe, is required. Raman spectroscopy is an accurate, specific, and rapid technique for identification, which has led to an exponential increase in portable and handheld Raman spectrometers. At present, there are over 30 vendors supplying portable Raman equipment to a range of sectors, such as first responders, hazmat operatives, military, explosive ordnance disposal, police, investigation teams, and customs. Differentiation between competing systems is generally limited to laser excitation wavelength (longer wavelengths, less interference from fluorescence), matching algorithms, software interface, and library content.
Conventional Raman has many advantages, one being the ability to identify contents within a clear glass vial or thin plastic bag. However, an opaque plastic or colored glass container results in a large fluorescent background, and can attenuate the beam. Spatially offset Raman spectroscopy (SORS) is a method developed at the Rutherford Appleton Laboratory, UK (1) that provides the ability to identify the contents of a truly opaque or complex container, without opening it to gain access or to take an aliquot of the sample (Figure 2). This capability provides several benefits, including safety (not disturbing the object or releasing materials into the environment), efficiency (time spent in personal protective equipment [PPE] is minimized), decision making (mission-critical data are obtained earlier in an operation), and the preservation of evidence and intelligence.
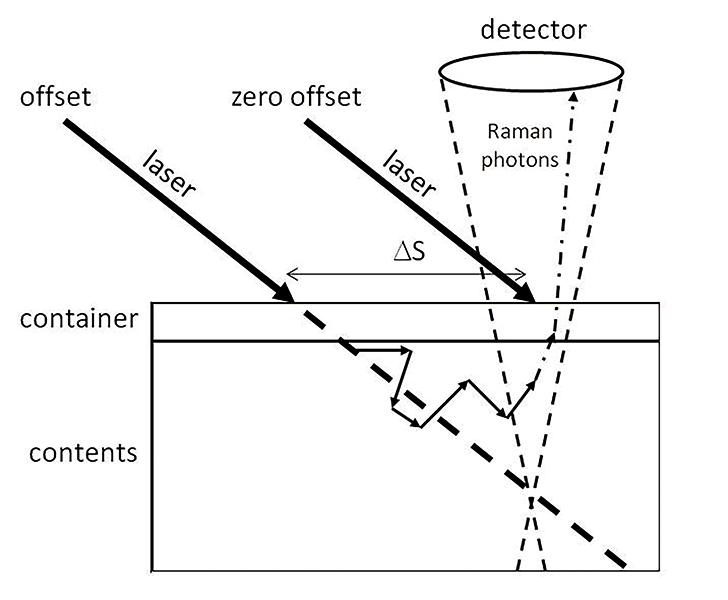
Figure 1: Schematic representation of SORS geometry showing the "zero offset" laser excitation relative to the detector, and the "offset" laser excitation, separated by a distance ΔS. Laser excitation generates scattered photons which have diffused through the container and contents. There is a change in Raman contribution from the container and its contents depending on offset measurement.
Raman spectroscopy relies on the inelastic (Raman) scattering of monochromatic light. Laser light excites molecular vibrations, resulting in a change in the energy of the scattered light shifted in wavelength from the excitation source. Subsequently, this produces a highly unique and characteristic spectrum of the molecules being analyzed, enabling Raman spectroscopy to be used to positively identify specific molecules of interest. The principle of SORS is based on taking multiple Raman measurements. First, a "zero offset" measurement is made; the detector and laser excitation is positioned in the same configuration as a conventional backscattered Raman system. This measurement typically consists of the container surface. Second, one or more measurements are then made with either the detector or the laser offset by a distance of ΔS (shown in Figure 1). This novel configuration allows for the detection of photons that have diffused further into the container. Therefore, the spectra obtained will consist of both the container and its contents. As the offset is increased, the container surface component of the signal depletes while the contents signal is increased. Acquiring multiple measurements results in varying signal contributions from both the container and its contents, making it possible to identify and separate the signal into its respective components. The process is fully automated, and does not require any additional intervention of the operator or prior knowledge of the barrier.
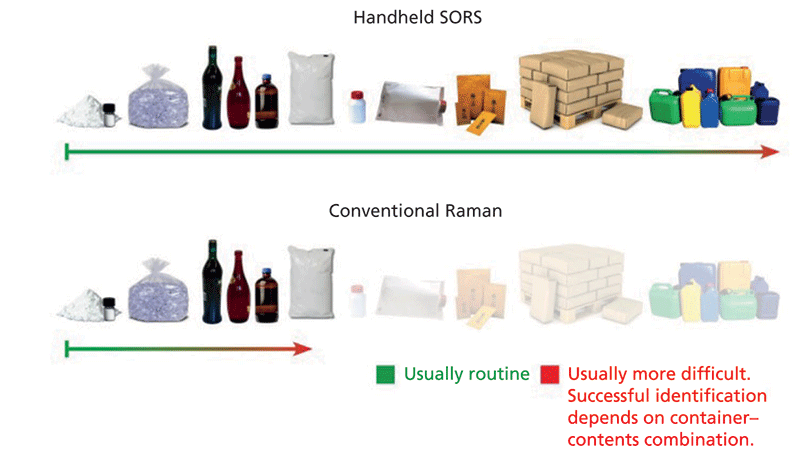
Figure 2: (Top): SORS extends through barrier giving the capability to work with a wide range of nonmetallic containers. (Bottom): Conventional Raman systems require line-of-sight and thus work with clear plastic bags and vials, and some translucent packaging.
Results and Discussion
A handheld SORS system (Resolve) was supplied by Agilent Technologies LDA UK limited, formerly Cobalt Light Systems (Oxford, UK), and used without any modification.
Explosive Materials
A major advantage of SORS technology is the ability to detect and positively identify the contents of a container through the barrier. In many parts of the world, improvised explosive devices (IEDs) are constructed from readily available materials, meaning explosive materials being transported may look like common benign household containers. A good example of this is yellow palm oil containers (YPOC), commonly used in many parts of Africa and Asia, used to make fuel-oxidizer type IEDs. The yellow, or sometimes blue, containers are typically made from molded high-density polyethylene (HDPE). This material attenuates the beam, adds a fluorescent background, and produces a strong Raman signal. Conventional Raman spectroscopy would not be able to scan effectively through these barriers. In the example shown in Figure 3, SORS successfully identified the mixture of ammonium nitrate and sugar from within the container, through the barrier wall, in < 2 min. Even more difficult is the ability to distinguish the "benign" version of this container; that is a YPOC which contains unprocessed (bright orange colored) palm oil. Difficulty arises from the fact that the contents are fluorescent, and the spectrum of many oil-like substances is very similar to HDPE making the processing challenge greater.
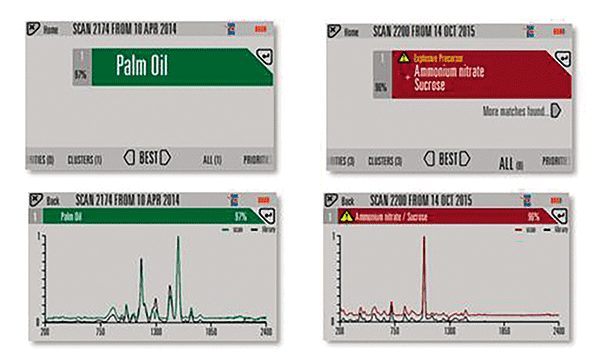
Figure 3: Example scans of West African yellow palm-oil containers with ammonium nitrate and palm oil contents, respectively. Items tagged as priorities in the software are displayed as red. A warning message (such as, "explosive precursor") is configurable by an authorized user.
SORS can also operate with a direct line of sight to a sample, such as exposed white powder or liquid spillage with a "surface scan" mode. Despite conventional Raman being a very effective technique, in practice, its use is often limited by operational procedures. The unique optical geometry used in this manifestation of SORS means the laser spot is not delivered coaxially with the collection path. This, in turn, results in a much larger laser spot, and, consequently, the power density of the laser on the sample is reduced (by orders of magnitude) compared to the tightly focused beam typical of a conventional Raman system. This is highly advantageous when scanning for sensitive explosives. Together with shorter exposure times (from high optical efficiency), there is a significantly reduced risk of detonation or deflagration, which improves user safety when working with sensitive explosives. Figure 4 shows an example of Raman spectra from nine primary and sensitive explosives. Sensitive explosives (approximately 2-5 g in each case) were scanned directly in point-and-shoot mode, or contained in a glass vial. Maximum laser power was used in each case (475 mW), and a timer delay was used to allow the operator to retreat to a safe distance.
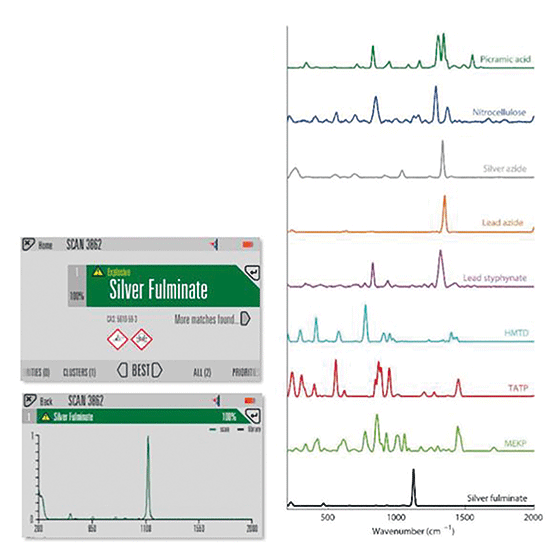
Figure 4: Right: processed SORS result and material identification. Left: example of silver fulminate scans. Scans are normalized in all cases.
The setup in Figure 4 was chosen to increase safety by not containing the sample, and to maximize laser power by not putting an attenuating barrier between the system and the explosive. Total measurement times in these modes were < 1 min at 100% laser power, and the scan timer delay function was used. Experiments were repeated 10 times and on several batches of the same material, with 100% detection and correct identification achieved. The high signal-to-noise ratio means that these sensitive materials can be accurately identified from the libraries of many thousands of chemicals.
Silver fulminate is a white or off-white powder which is highly sensitive to percussion or energy input and is known to detonate instantly when scanned using conventional Raman. Using SORS, the material could be identified in all scans with 100% accuracy and no deflagration or detonation. Care and caution should always be used when scanning sensitive explosive materials.
Narcotics and New Psychoactive Substances
The rise in the prevalence of hazardous narcotics and new psychoactive substances (NPS) presents a major challenge to law enforcement professionals. These materials vary in composition and color from pure white powders to raw and unrefined colored and brown samples. Many narcotics, and in particular NPS, are hazardous by inhalation, ingestion, or eye or skin contact. There are several worldwide reports that describe accidental exposure to officers, resulting in serious medical consequences. An example is fentanyl; this opioid is reportedly 10-1000 times more potent than heroin, with fatal doses comparable to a few grains of sugar. This material presents a high risk in its pure form (as it is smuggled or transported). Given the high potency of these substances, the risk remains after they are cut down into the multitude of street forms and products. This means there is a risk of exposure in situations where full protective equipment is not worn. Therefore, a technique that allows materials to be scanned through a variety of wrapping and packaging minimizes the risk of exposure to the operator.
Combining SORS, high quality data with a library that is continuously updated provides a powerful tool in detection of NPS. Many narcotics, especially those that are unrefined or cut with excipients, are fluorescent. Using a 830 nm laser excitation wavelength significantly reduces fluorescence emission in comparison to a commonly used 785 nm laser excitation. Excitation at 830 nm also produces significantly more Raman scattering than a 1064 nm system (λ-4 law), and allows the use of more sensitive detectors. Handheld SORS can identify narcotics and new psychoactive substances concealed within typical packaging, such as white and brown paper (fluorescent), plastic wrapping (Raman background), and green, brown, blue, or clear thick glass bottles (fluorescence).
The ability of handheld SORS to identify hazardous narcotics and NPS concealed behind barriers such as post packs, brown paper wrapping, cardstock, opaque polyethylene, opaque polypropylene, opaque PVC containers, and rubber or nitrile rubber has been tested at expert laboratories in the United Kingdom, United States, and China.
The SORS process can still operate successfully through multiple layers of differing composition without any prior knowledge. However, in cases such as these, the user must ensure that the nose cone is placed directly opposite and pressed towards the material to be scanned. The material needs to be within a maximum distance of ~5 mm of the nose cone for the SORS optics to function correctly. In cases where this distance is too great, the system will often report a plastic or no match.
Trace Test
SORS works very well with narcotics in concentrations from 100% down to ~10% w/w, such as those typical of smuggling operations and the raw material used in street product manufacturing. However, the concentrations of active component in some street samples is very low. This is especially true of fentanyl samples. In some instances, only trace (a few grains) of sample are readily available. This is not enough material to obtain a high quality Raman signal; therefore the sample must be concentrated, or the Raman scattering has to be enhanced or intensified. One method which enhances the Raman effect is surface enhanced Raman scattering (SERS). Metallic surfaces that have one dimension in the range of 1-100 nm exhibit interesting optical properties, which the corresponding bulk material does not. In the presence of metallic nanoparticles, typically gold or silver, a significant increase in sensitivity from this optical effect is achieved, an enhancement factor of 103-1012 has been reported, indicating that single molecule detection is possible (2). Not only do metallic nanoparticles enhance the Raman effect, the nanoparticle also quenches the competing fluorescence process (unrefined narcotics are typically fluorescent).
Using handheld SORS in connection with a trace detection kit also helps the user. For 2-3 mg of a mixture of 90:10 heroin to fentanyl (mixed with excipient this is typical of a street sample) was added to such a kit, and analysis was performed using 100% laser power (Figure 5). The presence of both fentanyl citrate and heroin was successfully identified. This amount of fentanyl material mixed with heroin would be extremely hard to detect in these quantities using conventional Raman. This trace kit test enables increased sensitivity of materials, hence detection of materials at lower concentrations. This is a significant step towards preventing and detecting chemical hazards and threats.
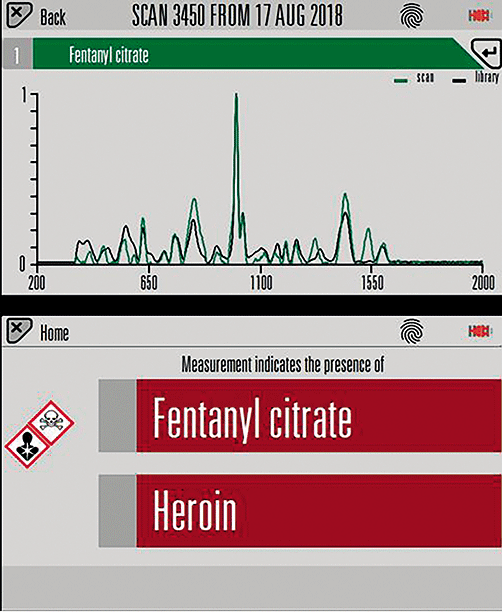
Figure 5: Results using handheld SORS showing the positive identification of fentanyl citrate and heroin found in a 2-3 mg sample.
Conclusions
A portable SORS system has been developed and is successfully deployed and utilized by military, first responders, customs, and law enforcement operators. Detection could be achieved in almost 100% of cases where the sample was concealed by a thick, opaque, or colored barrier with maximum scan times < 2 min. The examples presented represent a step change in the capability of portable Raman spectroscopy as these samples were previously inaccessible to the technique. A portable SORS system enables better information to be obtained earlier in an operation, and better decisions made without disturbing potentially hazardous materials (such as explosives or fentanyls). Hazardous materials and narcotics can be scanned from within the container, reducing the risk of accidental exposure to the operator.
References
(1) C. Eliasson, N.A. Macleod, and P. Matousek, Anal. Chem. 79 (21), 8185–8189 (2007).
(2) R Stokes, A Macaskill, P.J. Lundahl, W.E. Smith, K Faulds, and D Graham, Small3(9), 1593–1601 (2007).
Robert J Stokes, Ana Blanco, Rachel McGee, and Oliver Presly are with Agilent Technologies LDA UK Limited, in Abingdon, United Kingdom. Direct correspondence to: robert.stokes@agilent.com

Best of the Week: AI and IoT for Pollution Monitoring, High Speed Laser MS
April 25th 2025Top articles published this week include a preview of our upcoming content series for National Space Day, a news story about air quality monitoring, and an announcement from Metrohm about their new Midwest office.
LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.