Sample Introduction for ICP-MS and ICP-OES
Sample introduction can be a significant source of random and systematic error in the measurement of samples by inductively coupled plasma optical emission spectroscopy (ICP-OES) and ICP mass spectrometry (ICP-MS) systems.The considerations made in selecting a liquid introduction system include dissolved solids content, suspended solids presence, presence of hydrofluoric acid or caustic, detection limit requirements, precision requirements, sample load requirements, sample size limitations, and operating budget. The analyst is left with the task of choosing the best introduction components.This article discusses the key components of a typical liquid sample introduction system for inductively coupled plasma spectroscopy, and offers troubleshooting tips for problems commonly encountered by practitioners.
Sample introduction can be a significant source of random and systematic error in the measurement of samples by inductively coupled plasma optical emission spectroscopy (ICP-OES) and ICP mass spectrometry (ICP-MS) systems. Consequently, texts devoted to ICP have given sample introduction considerable attention (1–4). Samples most commonly are introduced as liquids. The considerations made in selecting a liquid introduction system include dissolved solids content, suspended solids presence, presence of hydrofluoric acid or caustic, detection limit requirements, precision requirements, sample load requirements, sample size limitations, and operating budget. The analyst is left with the task of choosing the best introduction components.
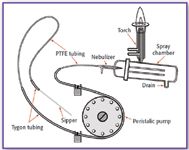
Figure 1. ICP liquid sample introduction system.
This article discusses the key components of a typical liquid sample introduction system, acquaints the reader with the basic options available, and addresses some common problems.
Pneumatic Nebulizers
The term "pneumatic" is defined as "of or relating to or using air or a similar gas." The word "nebulizer" is derived from the Latin "nebula," meaning mist and is defined as "an instrument for converting a liquid into a fine spray." Therefore, a pneumatic nebulizer is literally an instrument for converting a liquid into a fine spray that uses a gas as the driving force. The most popular types of ICP pneumatic nebulizers are concentric, fixed cross-flow, and high solids.
Concentric nebulizers. This basic type of nebulizer can be made from glass or plastics such as PFA and (depending upon design) is capable of handling a sample solution introduction rate of between 0.01 and 3 mL/min. Therefore, the "micro-concentric" nebulizer (0.01–0.1 mL/min) should be considered when sample size is limited to 1 mL. They typically are used with cyclonic spray chambers but can be used with the Scott spray chamber (an adaptor is needed in this case). Their capability of handling dissolved solids (type C better than type A) is dependent upon how near the dissolved solid is to its solubility limit. The glass construction should not be used with hydrofluoric acid or caustics such as the alkali hydroxides. Quartz construction is more resistant to chemical attack. The precision and detection limit capability of this design is excellent with both being very dependent upon the argon gas pressure going to the nebulizer. Although the sensitivity tends to vary from nebulizer to nebulizer, manufacturers have improved greatly upon their ability to reproduce this nebulizer. These nebulizers should never be used with solutions containing suspended solids. Although delicate, this nebulizer can be used for years with the proper care.
Cross-flow nebulizers. The cross-flow design began with a design that allowed for the adjustment of the two capillary tubes that are set at right angles to each other (5). The criticality of the capillary positioning led to the commercial production of the fixed cross-flow design. The fixed cross-flow is used with the Scott spray chamber, will provide moderate to good sensitivity, and generally is better than the concentric design for high solids. It is available in materials of construction that resist chemical attack. It is not as delicate as the concentric nebulizer and often is used in laboratories where the optimum in precision and detection limit is of less importance than having a large sample load capability. It is not recommended if suspended particles are present.
High solids nebulizers. High solids nebulizers come in a variety of designs. The V-groove design is very resistant to salting out and can be used when suspended particles are present. Some designs require relatively high gas pressures, which might present some difficulty. Designs include materials of construction that resist hydrofluoric acid and caustic attack. In general, expect moderate detection limit and measurement precision. This design is used where high and suspended solids are an every day occurrence.
Ultrasonic Nebulizers
Sound can be used instead of a gas as the energy source for converting a liquid to a mist. These nebulizers use an ultrasonic generator at a frequency of between 200 kHz and 10 MHz to drive a piezoelectric crystal. A pressure is produced that breaks the surface of the liquid–air interface. Ultrasonic nebulizers are significantly more expensive than the pneumatic designs but they will improve (that is, lower) detection limits roughly by a factor of 10.
Spray Chambers
The most common types of spray chambers are the Scott double-pass and the cyclonic, the latter of which is relatively new but is very popular. The purpose of the spray chamber is to remove droplets produced by the nebulizer that are >8 µm in diameter. Important considerations here include the wash-in time, wash-out time, stability, and sensitivity. The drainage characteristics are important in part due to pressure changes that can occur during drainage. It is important that the drainage process be smooth and continuous. The analyst might observe faster wash-in and wash-out times with the cyclonic design. The chamber material of construction, chamber surface area, volume, and flow pattern as well as the sample matrix and the chemistry of the element influence the wash-out times. In addition, the analyst might observe faster wash-out times with glass construction than with polymers due in part to better wetability of the glass (lack of beading).
Torches
The two basic designs are the Greenfield and Fassel torches. The Greenfield torch requires higher plasma gas flow and radio frequency power than the Fassel. Both designs produce similar detection limits. The Greenfield torch is more rugged (less likely to extinguish due to misalignment, and introduction of air) whereas the Fassel torch requires lower argon flow and less RF power. Some nebulizer designs work better with one torch design than another. Before experimenting with torches it is best to contact your instrument manufacturer to determine the torch design recommended for your instrument and any design specifications, operating conditions and dimensions that must be observed.
Questions to Consider
The following are some questions you might want to consider with regard to these sample introduction components, whether you are purchasing a new ICP unit or already have one or more existing units:
- What torch design is used and what are the power and argon gas flow requirements? (You might want to calculate your annual argon expense.)
- What nebulizer and spray chamber designs are available and can they be obtained from alternate suppliers?
- Are there specific nebulizer designs that cannot be used with the torch–spray chamber that is recommended or required?
- What are the costs of the individual introduction system components and how much expense would be expected or actually occurs throughout a year of operation?
- What is the lifetime of the torch and what is the most common reason for failure?
- How tolerant is the system to slight changes in torch alignment?
- How tolerant is the system to air coming from the nebulizer? Will it extinguish after a few seconds?
- How tolerant is the system to the introduction of organic solvents?
- What is the lowest boiling point solvent that can be introduced?
- How tolerant is the system to the torch building up coke for aromatic and aliphatic nonpolar solvents?
- What is the most precise nebulizer that can be used with your ICP system and what precision should be obtained?
- What are the detection limits for your analytes of interest? Are you achieving the detection limits required for your application?
- When looking for lower detection limits have you considered axial view ICP? Ultrasonic nebulizers? Both?
- Do your analytical solution samples contain high levels of dissolved solids? Any suspended solids?
- Do you experience nebulizer salting out? Plugging?
- Which high solids nebulizer is recommended for your current or potential ICP, and what is the precision to be expected?
- How rugged is it and what does it cost?
- How difficult is it to connect an ultrasonic nebulizer?
- Can either the Scott or cyclonic spray chamber designs be used?
- What are the washout times for mercury, boron, yttrium, and copper in nitric acid? In hydrochloric acid?
- Are corrosion-resistant, hydrofluoric acid–resistant introduction systems available? What do they cost? How easy are they to switch in and out?
- When analyzing for silicon in trace hydrofluoric acid how much of a silicon, boron, sodium, and aluminum background signal do you get? If the introduction system contains glass- how much hydrofluoric acid can be tolerated before signals from silicon are observed? Before damage occurs?
Avoiding Connection Problems
The key elements of a sample introduction system start with the sipper tube and end with the torch (Figure 1). They are listed as follows:
- Sipper – typically plastic
- PTFE tubing going from the sipper to the peristaltic pump tubing
- Peristaltic pump tubing
- PTFE tubing going from the peristaltic pump tubing to the nebulizer
- Spray chamber
- Torch
The main difficulty that analysts typically experience with introduction system failure is that of connections between components. The connections are listed as follows:
- Sipper to PTFE tubing
- PTFE tubing to peristaltic tubing (both into and out of)
- PTFE tubing from peristaltic pump to nebulizer
- Nebulizer to spray chamber
- Spray chamber to waste drain tube
- Spray chamber to torch
If any one of these connections is not air tight the operator will experience anything from poor precision to an inability to light the plasma. One of the reasons many people prefer concentric glass nebulizers is that they are "free flow;" that is, the liquid will flow from the sample container to the nebulizer without assistance from the peristaltic pump. A simple check is to determine if you obtain a fine steady mist — using water as the sample — without the peristaltic pump (pressure lever released) so that free flow can occur. This can be done with the nebulizer disconnected from the spray chamber (plasma has not yet been lit) so that the mist can be visualized easily. You also can check for the appearance of any small air bubbles in the PTFE tubing; bubbles should never be present and indicate a poor connection somewhere between the sipper and the nebulizer.
A connection that often is taken for granted is the spray chamber drain–waste tube connection. This connection is absolutely critical. One way to test it is to put some water in the spray chamber using a wash bottle and determine if it drains smoothly and without leaks. Poor precision or the inability to light the plasma is a common symptom of a poor drain tube connection. During this test you also should observe the absence of water droplets in the spray chamber (assuming glass construction). A dirty spray chamber will leave water droplets and cause poor precision and carryover problems. Make sure the plasma is not lit when you perform this test.
Cleaning Components
Glass or quartz components often are preferred because of their ease of operation and cleaning. It is always best to start the day with a clean nebulizer, spray chamber, and torch. Cleaning the torch daily also will extend its life. Many types of cleaning solutions can be used. Some of our analysts prefer 1:1 nitric acid and water and others prefer sulfuric acid and hydrogen peroxide, while others prefer 1:1 hydrochloric acid–nitric acid. All of these cleaning solutions work depending upon the nature of the contaminants. The sulfuric–peroxide is needed if organics such as grease are suspected. Ultrasonic baths are great but never use them to clean a glass concentric nebulizer. Glass concentric nebulizers are cleaned by leaching and occasionally by applying a backpressure with water to remove lodged particles. The use of a cleaning wire or ultrasonic bath is a sure way to destroy the nebulizer.
Spray chambers come in all glass, all plastic and glass with plastic end caps. If you do not use hydrofluoric acid (all plastic systems are needed with hydrofluoric acid) and therefore have the luxury of using glass components attempt to use a spray chamber without the plastic end cap. They typically are used with glass concentric nebulizers and use only two O-rings to connect the nebulizer to the spray chamber. The plastic end cap can cause longer wash-out times and carryover problems, and presents a very large connection surface where connection problems can occur. Using a glass concentric nebulizer and all glass spray chamber, a precision of between 0.2 and 0.5% RSD should be observed. If an all-glass system gives a precision of 0.6 to 1% RSD or greater then there is most likely a connection problem or the nebulizer gas flow rate is too high (look for spitting when checking the nebulizer free flow and do not be afraid to lower the gas pressure to the nebulizer).
Pump Tubing
Another weak link in the introduction system is the peristaltic pump tubing. When you start the day the tubing is fresh and the pressure can be set to give a steady mist when the pump in running. The problem is that the pump tubing stretches and either the nebulizer argon pressure is not enough to drive the solution through the tubing or over tightening occurs, resulting in a pulsating mist spray. This is a problem that each analyst has to be aware of and solve through experimentation. This problem is particularly troublesome for ICP-MS users because the sample flow rate decreases as the tubing stretches, causing a relative increase in the sensitivity ratio of the higher: lower atomic number elements. An option the ICP-MS operator might choose to explore is free flow (no pump) using a micro-concentric nebulizer.
In summary, when it comes to ICP introduction systems there is no substitute for experience, so try different approaches.
Paul Gaines is senior technical advisor with Inorganic Ventures (Lakewood, NJ; www.ivstandards.com).
References
1. H.E. Taylor, Inductively Coupled Plasma Mass-Spectrometry, Practices and Techniques (Academic Press, New York, 2001).
2. Inductively Coupled Plasma Mass Spectrometry, A. Mantaser, Ed. (Wiley-VCH, New York, 1998).
3. Inductively Coupled Plasmas in Analytical Atomic Spectrometry, A. Montaser and D.W. Golighty, Eds. (VCH Publishers, New York, 1992).
4. M. Thompson and J.N. Walsh, A Handbook of Inductively Coupled Plasma Spectrometry (Blockier London, U.K., 1983).
5. R.N. Kniseley et al., Applied Spectroscopy 28, 285–286 (1974).

Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.