Single-Cell Analysis by Inductively Coupled Plasma–Time-of-Flight Mass Spectrometry to Quantify Algal Cell Interaction with Nanoparticles by Their Elemental Fingerprint
This method detects elements intrinsically present in cells, and because sc-ICP-TOF-MS measures a full mass spectrum, no analytes are missed.
Single-cell analysis has recently become a growing research field, and has found many applications in a variety of disciplines such as biochemistry, toxicology, metallomics, and even medical diagnosis and pharmaceutics for drug and cancer research. Although several analytical methods exist for the analysis of individual cells and their cellular content, this article focuses on highlighting the latest applications and trends in which inductively coupled plasma–time-of-flight mass spectrometry (ICP-TOF-MS) is applied to single-cell analysis. The novelty of this method relies on the determination of the elemental fingerprint through the detection ofelements intrinsically present in cells. By always measuring a full mass spectrum, there is no need for compromising which analytes are measured, and nothing is missed. This is particularly beneficial for nanotoxicology studies, where a direct and complete picture of the nanoparticle–cell association is provided.
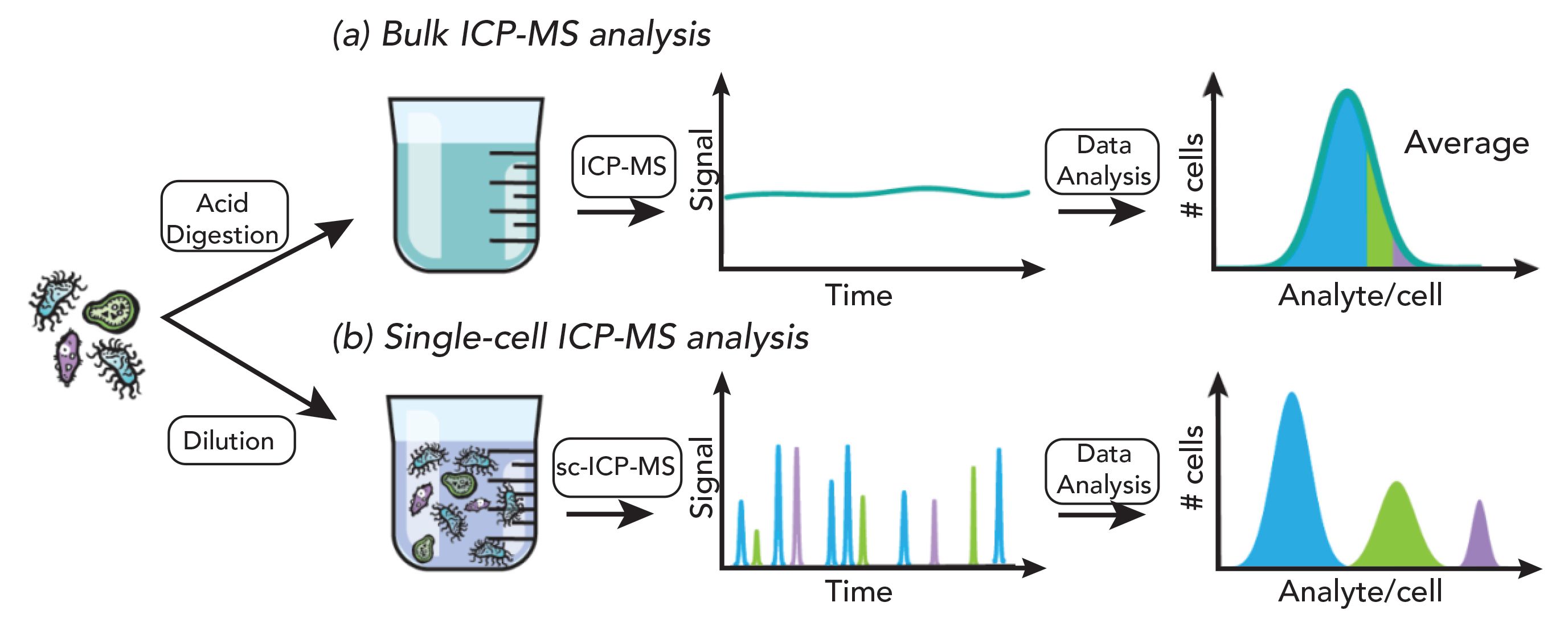
The conventional approach to determine the bioavailability of metals in cells involves the acid digestion of a cell pellet and a subsequent analysis with inductively coupled plasma–mass spectrometry (ICP-MS). This procedure has the drawback of requiring a large number of cells, and only provides an average value for a given cell population (1). However, cells are known to be heterogeneous (2), and only their analysis on an individual basis using single-cell ICP-MS will make it possible to obtain information related to their heterogeneity (see Figure 1). Single-cell ICP-MS (sc-ICP-MS) is comparable to single-particle ICP-MS (sp-ICP-MS); indeed, both techniques rely on the same fundamental principles and the capability to measure data at high time resolution, thereby allowing the acquisition of time-resolved transient signals produced by single entities, such as (but not limited to) nanoparticles (NPs) or cells. Since the first seminal paper of Degueldre and Farvagner in 2003 establishing the basics of sp-ICP-MS (3), the last two decades have witnessed a rapid maturation of the technique through the development and commercialized of improved hardware and dedicated software for data processing, as well as by a continuously growing number of publications. The research and efforts invested in sp-ICP-MS have simultaneously benefited single-cell analysis by ICP-MS.
Figure 1: (a) After acid digestion of the pelletized cells, the resulting solution is introduced into the ICP-MS by conventional nebulization, where a steady state signal is recorded. In this bulk method, an average value is obtained for the thousands of cells contained in the initial pellet, masking the stochastic diversity of individual cells, and assuming a homogeneous population. Consequently, minority cell populations (represented by the colors green and purple), exhibiting differences in their elemental composition compared to the majority cell population, will pass unnoticed, and perfectly illustrates Simpson’s paradox. (b) In single-cell ICP-MS analysis, a dilute suspension of cells is introduced into the plasma. Each cell produces a distinct ion cloud, recorded as a signal spike. This approach enables the detection of each individual cell, and thereby guarantees the conservation of cell variability information. By analyzing cells on a cell to cell basis, three distinct populations based on their different analyte content can be recognized rather than yielding an average.
In sc-ICP-MS, the cells are carried in droplets generated by nebulization of the suspension into the plasma, where they are successively vaporized, atomized, and finally ionized. Each cell produces an ion cloud, which is detected as an individual spike above the background. Similarly, as in sp-ICP-MS, the frequency of the recorded spikes is proportional to the cell number concentration, and the magnitude of these spikes relates to the cell’s element mass. The applicability of this technique was successfully demonstrated by determining magnesium levels in algae (4), and was further applied in nanotoxicology studies assessing the cellular uptake of NPs (5–7).
Although the measurement strategy behind single-cell ICP-MS appears straightforward, implementation to obtain reliable data can be quite challenging. Besides high background from the culture media and cell fractionation in the sample introduction system, a major bottleneck repeatedly reported in single-cell studies is inefficient transport of the cells because of their larger sizes, when compared to NPs. Indeed, conventional systems, which usually consist of a cyclonic spray chamber, are designed for the introduction of smaller droplets of solution, and result in transport efficiencies of less than 10%. Tailored systems for single-cell introduction, including improved nebulizers or full consumption spray chambers (8,9), as well as innovative setups (10,11) have been developed and tested over the years to ensure a high transport efficiency of intact cells into the ICP, and are key for successful analyses. Another limitation relies in the performance of the mass analyzer; conventional ICP-MS instruments featuring a quadrupole or sector-field mass analyzer are restricted to the simultaneous detection of one to two isotopes at most. This is particularly disadvantageous when trying to quantify the association of NPs and cells in nanotoxicology studies. To obtain the full picture, a simultaneous mass analyzer, such as time-of-flight, is beneficial.
Time-of-Flight Technology
In time-of-flight mass spectrometry (TOF-MS), the fundamental principle is based on the separation of ions based on their flight time through a flight tube with known length before reaching the detector. Having the same kinetic energy after a pulse acceleration voltage, light ions will travel faster than heavier ones. The measured arrival times of all ions yields a time spectrum that, after simple calibration, can be converted into a mass spectrum. Major advantages of TOF mass analyzers include no limitations on the number of isotopes analyzed, and fast data acquisition. The latter is particularly important when dealing with discrete entities such as single cells, which produce short transient signals < 1 ms.
However, it should be noted that TOF technology is not a new concept in the field of single-cell analysis, and was initially introduced in 2009 by Bandura, with a prototype instrument (12) for time-resolved analysis of individual cells, thereby opening the door for the development of “mass cytometry” as a new field. Mass cytometry instruments are ICP-TOF-MS instruments redesigned for specific single-cell applications (13).The principle of this application is to use stable rare earth metal isotopes to label cells, thereby allowing their detection through their metal marker (14). Similar to fluorescence-based flow cytometry, this labeling procedure enables a multiparametric analysis of single cells. Beside a big interest in biological research and drug screening applications, mass cytometry has also been used to detect silver NPs in bacteria cells (15). The detection of many intrinsic elements is, however, not possible with mass cytometry, because of a limited mass range (>80 Da) and sample preparation procedures that involve staining.
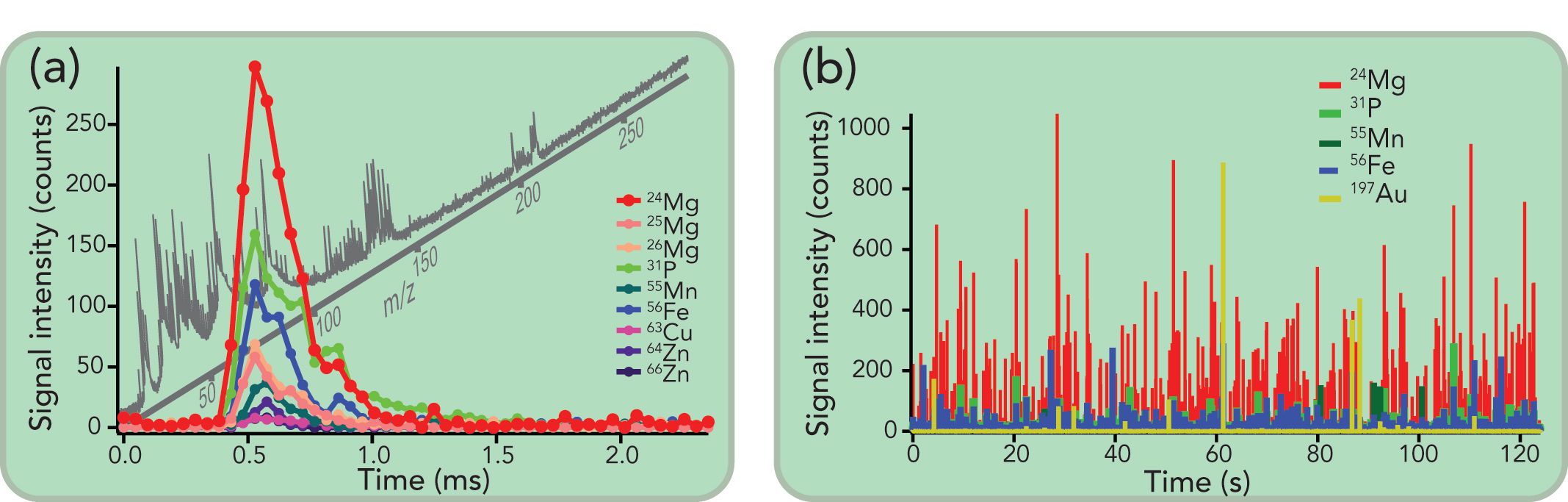
ICP-TOF-MS can measure a full mass spectrum ranging from m/z = 14 to 280 (16), thereby allowing to detect light mass elements such as Na, Mg, P, S, K, Ca, Mn, Fe, Cu, and Zn. These elements are intrinsic elements of a living cell, and their distribution (also referred to as the cell ionome [17]) can provide insights into the development state of the cell (18). For example, phosphorous is found in nucleic acids (DNA and RNA), and also is an important constituent of energy compounds in cells, such as adenosine triphosphate (ATP), cytidine triphosphate (CTP), guanosine-5’-triphosphate (GTP), and uridine-5’-triphosphate (UTP). Sodium and potassium are active in the transmission of electrical signals, and zinc is used as a catalyst by several enzymes in various biological processes. Thanks to its simultaneous multi-element detection capabilities, ICP-TOF-MS can enable fingerprinting based on the correlation analysis of multiple elements (19). As illustrated in Figure 2a, magnesium, phosphorous, manganese, iron, copper, and zinc were identified as fingerprint elements of the analyzed algae. Consequently, no labeling nor staining is required, as the cells are detected on the basis of their “natural” elemental fingerprint (20,21). Moreover, a more specific fingerprint can be acquired by measuring metallic micronutrients that are specific for a certain cell type. Algal cells are rich in metallic micronutrients, such as Mg, which is a core building block for chlorophyll pigment, vital for photosynthesis. As a result, the metallic micronutrient composition can be used as a unique fingerprint to clearly identify different cell species. By measuring metal atoms at the cellular level, the goal is to achieve a better understanding of essential biological processes regulated by metalloproteins and metalloenzymes, thereby unlocking the keys to differentiate different states of a cell’s life cycle (22). Although the biochemistry of a cell is not utterly reflected by its ionome, deeper insights into the cell’s conditions and biological processes inside a cell can definitively be gained by monitoring changes in the cell’s metal content.
Figure 2: (a) Transient signal of a raphidocelis subcapitata (a freshwater algal cell), acquired with a time resolution of 48 µs. For each data point, a full mass spectrum is recorded, allowing the determination of the cellular fingerprint, which consists of Mg, P, Mn, Fe, Cu, and Zn. (b) Algal cells are commonly used in toxicological risk assessment studies, and were analyzed here after exposure to Au NPs to investigate their uptake. The simultaneous detection over the full mass range of the ICP-TOF-MS instrument allows the tracking of cells based on the detection of their elemental fingerprint, and enables a direct quantitative measure of the NP-cell association.
By using a TOF mass analyzer as a detector, a full mass spectrum is systematically acquired, allowing for the characterization of the environment in which a specific entity is found. Therefore, in the context of nanotoxicology, one can easily determine whether NPs are associated with cells or not.
Application Example of Single-Cell Analysis by ICP-TOF-MS
The analysis of single entities, which produce mass-limited signals as opposed to bulk measurements, requires higher sensitivity. Here, the capabilities of the specific ICP-TOF-MS (icpTOF S2, TOFWerk, Switzerland) used for the analysis of single entities (such as single cells or NPs), are highlighted in the following case study.
Following the rapid increase in the manufacture and widespread use of NPs, nanosafety and nanotoxicology have been topics of intensive research for the past two decades. An important parameter in safety assessment studies is the analysis and quantification of the cellular uptake.
Owing to their high spatial resolution, transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are often used for the localization of NPs in cells (23,24). Nevertheless, despite the impressive imaging capabilities of TEM and SEM, a major drawback of electron microscopy-based methods is the extensive sample preparation required. Furthermore, the obtained information is qualitative, and can be difficult, as well as very time-consuming, to interpret without additional elemental quantification or automated image analysis (25,26). As introduced previously, single-cell ICP-MS can also be used to quantify cellular uptake of NPs, providing information regarding the number of NPs associated with cells based on the magnitude of the observed spikes (5,6).
Three distinct observations are then typically expected from single-cell experiments using sc-ICP-TOF-MS and sc-ICP-MS:
- detection of only NP elements,
indicating the presence of NPs in the solution - detection of only cell elements without any NP elements, suggesting that no NPs were associated with the cells
- simultaneous detection of cell elements and NP elements, implying that NPs were associated with the cells.
Depending on the frequency and the magnitude of the associated NPs observed, the percentage of cells associated with NPs, as well as an estimate of the number of NPs associated with each algal cell, can be determined. In ideal cases, a dynamic evaluation of the possible increase of NP associated with algal cells as a function of both concentration and exposure time is performed.
In the present case study, algal cells were exposed to BaSO4 (NM-220) for a period of 72 h, and subsequently washed following the procedure proposed by Merrifield and associates (5), thereby removing unbound NPs. After exposure and subsequent rinsing in ISO 8692 algal media (27), the samples were expected to contain exclusively algal cells with associated NPs, and were stored in 15 mL falcon tubes wrapped in aluminum foil until analysis.
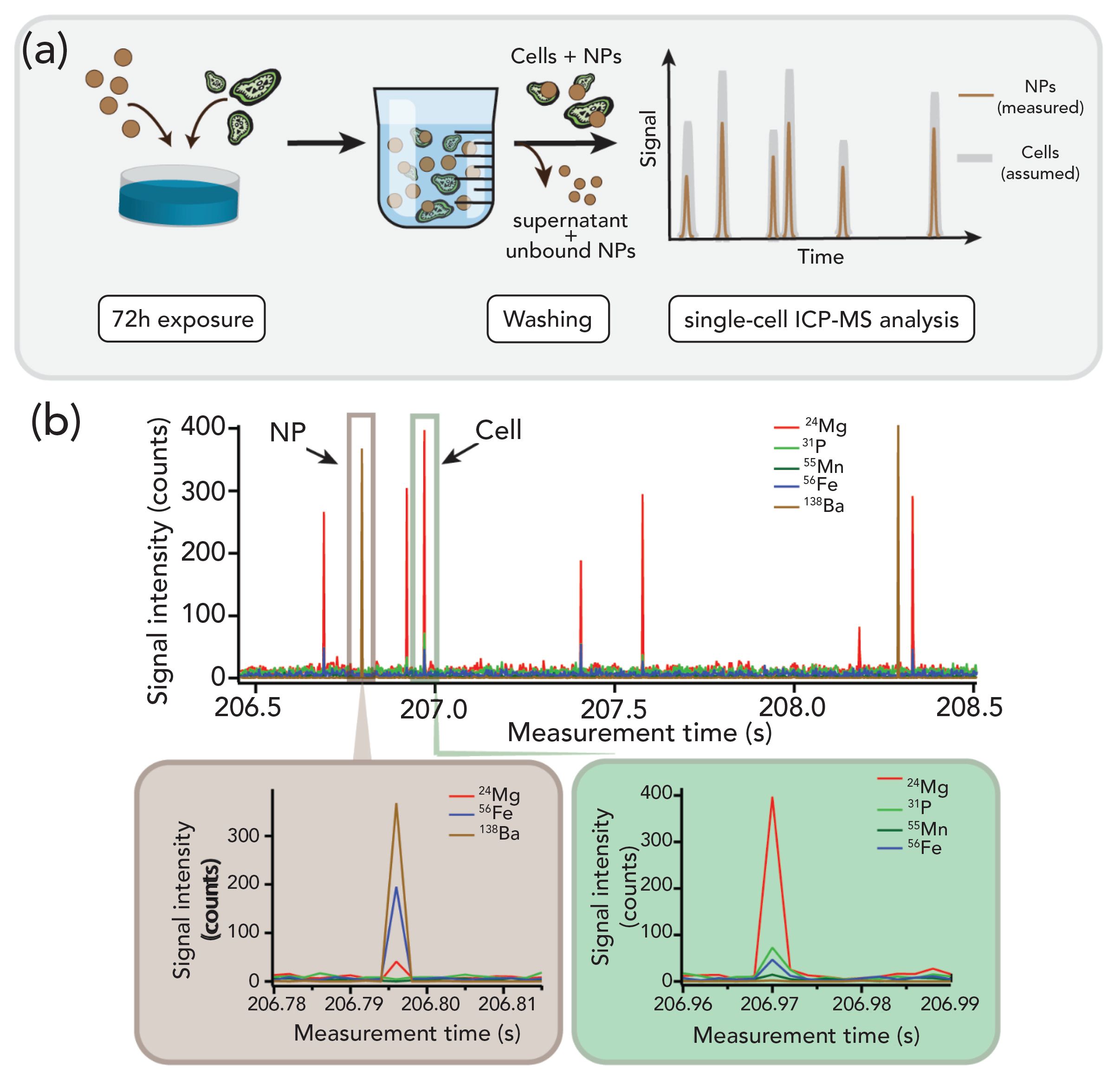
Initial studies using a quadrupole ICP-MS instrument setup for sc-ICP-MS showed the presence of BaSO4 NPs in the washed cells suspension, which were proposed to be associated with algal cells as illustrated in Figure 3a). However, it was not possible to confirm this hypothesis as only one element, 138Ba, was measured. Consequently, a similar experiment was performed using single-cell ICP-TOF-MS (see Figure 2a). As the elemental fingerprint of the analyzed algae cells is already known from Figure 2a, only events for which Mg, P, Mn, and Fe were detected concurrently, were attributed to algae cells. Surprisingly, analysis of the samples using sc-ICP-TOF-MS showed no association of BaSO4 NPs with the fingerprint signals from the algae cells after exposure for 72h as displayed in Figure 3b). Moreover, the barium signals were detected concurrently with traces of Mg and Fe. It should be emphasized that the Ba signals were exclusively observed along with Mg and Fe signals but none of the other constituents of the algae fingerprint were seen. Although it is possible that the missing signals were below the limit of detection, the observed elements did not match previously observed fingerprints for the algal cells. Without the multiple element detection, the detection of NP signals in the washed cells suspension step could easily be misinterpreted as NP association with algal cells. However, when using ICP-TOF-MS it becomes evident that the BaSO4 NP signals were not associated with the algal cell.
Figure 3: (a) Schematic representation of the experiment. After a 72 h exposure period, the cells are washed, allowing the removal of the supernatant containing the unbound NPs. The resulting cells pellet is resuspended in solution, and analyzed by ICP-MS in single-cell mode, under the assumption that the detected NPs are associated with the cells. (b) By repeating the single-cell ICP-MS measurements with a TOF mass analyzer, the veracity of the previous assumption can be directly assessed. By tracking the cells through their elemental fingerprint, it is possible to directly verify the concurrence of NP signals and cell signals. Results showed no direct association between NPs and cells, but concurrence of Ba signals with Mg and Fe signals.
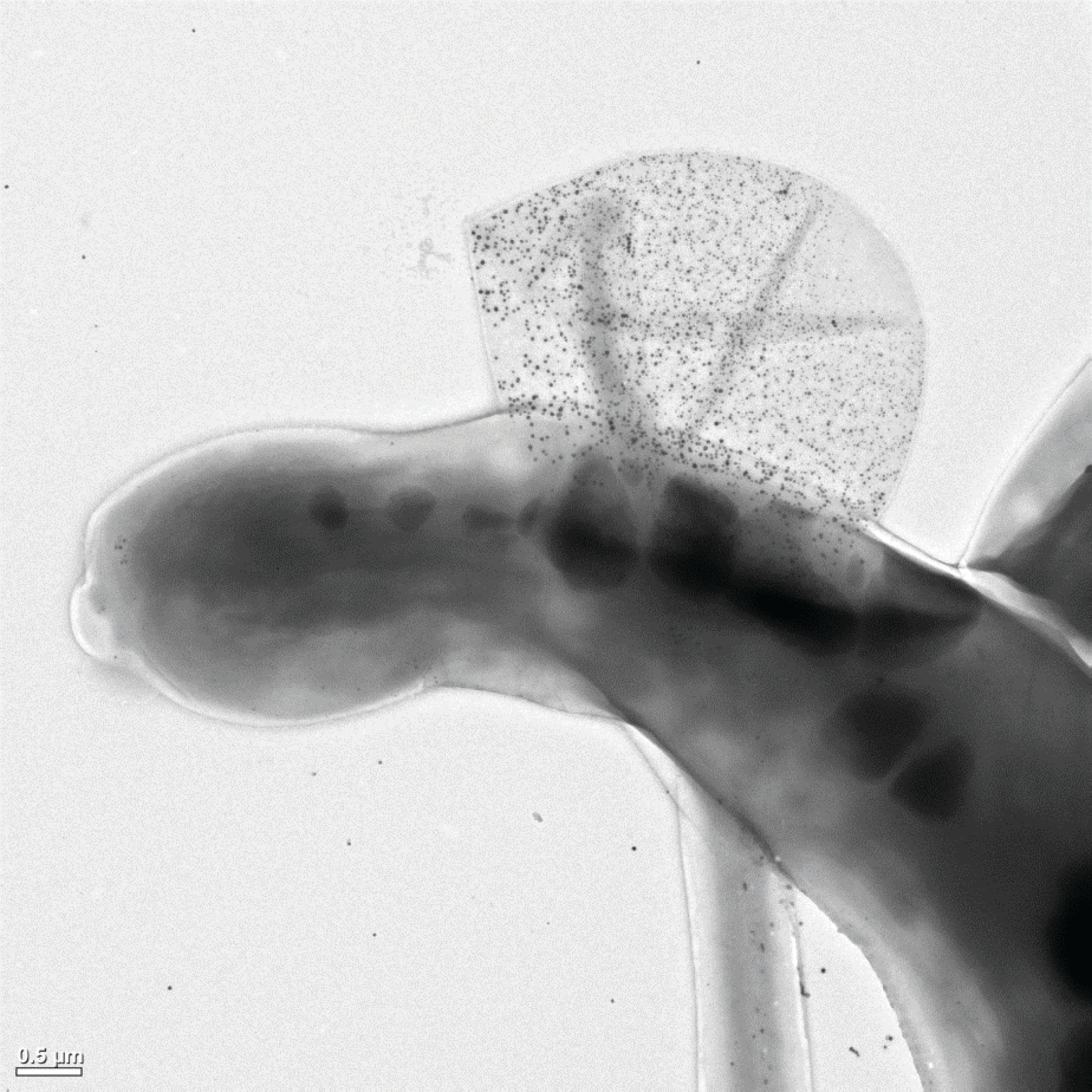
These findings triggered the discussion of new possible mechanisms explaining the observed phenomenon. A plausible explanation is the clearance of NPs adhered to the surface of algal cells by, for example, release of extracellular polymeric substances (EPS). EPS have been proposed to be a key factor for the bioavailability of NPs in relation to algal cells (28,29). An increased production of EPS would enable the algal cells to shed NP, thus actively mitigating uptake or adsorption to the exterior of the cell, while the NP would still be present in the sample, in the form of EPS containing NP. Although quantitative data on this behavior is lacking, this could explain the association of the BaSO4 NPs with Mg and Fe. The presence of Fe concurrent with the NP can be explained through complexation of dissolved Ba with EDTA from the ISO 8692 media, added to keep Fe in a bioavailable form. Using TEM as complementary technique, EPS clusters containing NPs can be observed, as shown in Figure 4. However, the exact mechanism and the frequency of the behavior are difficult to quantify, due to the qualitative analysis and the delicate nature of the EPS structures. Sc-ICP-TOF-MS would enable direct quantitative analysis of the phenomenon without the need to embed the samples, while also analyzing a larger number of algal cells or EPS clusters within a relatively short time span, providing robust statistics. Additionally, sc-ICP-TOF-MS could be used dynamically to sample from an algal suspension to evaluate the frequency of this clearance behavior as a function of concentration and time to further understand the interaction between algal cells and NP. This line of thought using ICP-TOF-MS to study the dynamic uptake and clearance behavior as a function of time and concentration is not only limited to algal cells, but could be expanded to other cell types, such as mammalian cells or bacteria used in nanomedicine or nanobiotechnology.
Figure 4: Transmission electron microscopy image of an algal cell (Raphidocelis subcapitata) previously exposed to silver NPs, shedding extracellular polymeric substances containing the alleged silver NPs. (Image courtesy of Louise H. S. Jensen and Sara N. Sørensen).
As highlighted through this study, although conventional sc-ICP-MS using quadrupole MS can be used to measure cells on an individual basis, it is limited to the simultaneous measurement of one or two isotopes at most. Consequently, even if NP signals are detected, no direct association with cells can be established. This information is crucial in determining whether the NP is, in fact, interacting with the algal cell. Further TEM analysis is then required to determine whether the NP is taken up by the algae, or adsorbed to its exterior. Additionally, using ICP-TOF-MS, the ionome of the exposed algae can be compared with that of control algae, allowing the assessment of their condition. This information is extremely valuable for a mechanistic understanding of algal cell interaction with NP. It could further facilitate the development of physiologically anchored tools for NP risk assessment by assessing precursors for stress responses experienced by the algal cell prior to the expression of classical endpoints, such as inhibition of population growth.
Conclusion and Future Direction
Sc-ICP-TOF-MS is a new, exciting, and fast-growing research field, which will still benefit from a few years before reaching the level of mass cytometry in multiparametric analysis of single cells. Thanks to its improved sensitivity and simultaneous full mass spectrum detection capability, ICP-TOF-MS has the capability to detect untagged cells based on their elemental fingerprint, thereby allowing for new creative experimental designs. For example, in addition to measuring the direct association of NPs and cells, the multi-elemental data recorded by ICP-TOF-MS may be used to assess the different states of cells in response to NP-mediated toxicity.
Alternatively to liquid sample introduction, single-cell analysis can also be performed using laser ablation (LA)-ICP-TOF-MS (30,31). By applying the cells on a sample holder and rastering over the sample with a laser, a two-dimensional image of the elemental distribution across the cells is produced, where each pixel contains a full mass spectrum. The elemental distributions observed in the cells provide additional insights into various phenomena, such as uptake, accumulation, and release on a single-cell level. The high spatial resolution of LA-ICP-TOF-MS images is particularly interesting for nanotoxicology studies, as it enables sub-cellular localization of NPs and the determination
of aggregates (22).
Furthermore, the large amount of data generated can be overwhelming; therefore, it is advisable to apply dimensionality reduction techniques, such as principal component analysis (PCA), commonly performed in mass cytometry workflows. Machine learning tools could help reduce the dimensionality the data, and extract information relative to the cell state and type, thereby making the classification of the data easier. Either way, continued efforts to improve this technique, followed by application- driven studies that are not limited to nanotoxicology studies, but that can be extended to metallomics and cell biology, are the necessary steps to unveil the potential of ICP-TOF-MS for single-cell analysis.
Acknowledgments
The authors thank Olga Meili and Aiga Mackevica for taking time to proofread this article and providing feedback. Lars M. Skjolding was funded by PATROLS–Advanced Tools for NanoSafety Testing, Grant agreement 760813 under Horizon 2020 research and innovation program. Thanks to Louise Helene Søgaard Jensen and Sara Nørgaard Sørensen for permission to use the TEM image presented in Figure 4. Finally, a special thanks to Robert Thomas for the invitation to contribute to his “Atomic Perspectives Column” in Spectroscopy.
References
- S.J. Altschuler and L.F. Wu, Cell 141, 559–563 (2010).
- W.M. Elsasser, Proc. Natl. Acad. Sci. USA 81, 5126–5129 (1984).
- C. Degueldre and P.Y. Favarger, Colloids Surf. A 217, 137–142 (2003).
- K.S. Ho and W.T. Chan, J. Anal. At. Spectrom. 25, 1114–1122 (2010).
- R.C. Merrifield, C. Stephan, and J.R. Lead, Environ. Sci. Technol. 52, 2271–2277 (2018).
- F. Abdolahpur Monikh, B. Fryer, D. Arenas-Lago, M.G. Vijver, G. Krishna Darbha, E. Valsami-Jones, and W.J.G.M. Peijnenburg, Environ. Sci. Technol. Lett. 6, 732–738 (2019).
- I.L. Hsiao, F.S. Bierkandt, P. Reichardt, A. Luch, Y.J. Huang, N. Jakubowski, J. Tentschert, and A. Haase, J. Nanobiotechnology 14, 1–13 (2016).
- A.S. Groombridge, S.I. Miyashita, S.I. Fujii, K. Nagasawa, T. Okahashi, M. Ohata, T. Umemura, A. Takatsu, K. Inagaki, and K. Chiba, Anal. Sci. 29, 597–603 (2013).
- M. Corte-Rodríguez, R. Álvarez-Fernández García, P. García-Cancela, M. Montes-Bayón, J. Bettmer, and D. Kutscher, Curr. Trends Mass Spectrom. 18, 6–10 (2020).
- K. Shigeta, H. Traub, U. Panne, A. Okino, L. Rottmann, and N. Jakubowski, J. Anal. At. Spectrom. 28, 646–656 (2013).
- P.E. Verboket, O. Borovinskaya, N. Meyer, D. Günther, and P.S. Dittrich, Anal. Chem. 86, 6012–6018 (2014).
- D.R. Bandura, V.I. Baranov, O.I. Ornatsky, A. Antonov, R. Kinach, X. Lou, S. Pavlov, S. Vorobiev, J.E. Dick, and S.D. Tanner, Anal. Chem. 81, 6813–6822 (2009).
- K.R. Atkuri, J.C. Stevens, and H. Neubert, Drug Metab. Dispos. 43, 227–233 (2015).
- S.D. Tanner, V.I. Baranov, O.I. Ornatsky, D.R. Bandura, and T. C. George, Cancer Immunol. Immunother. 62, 955–965 (2013).
- Y. Guo, S. Baumgart, H.J. Stärk, H. Harms, and S. Müller, Front. Microbiol. 8, 1–9 (2017).
- L. Hendriks, A. Gundlach-Graham, B. Hattendorf, and D. Günther, J. Anal. At. Spectrom. 32, 548–561 (2017).
- M. Malinouski, N.M. Hasan, Y. Zhang, J. Seravalli, J. Lin, A. Avanesov, S. Lutsenko, and V.N. Gladyshev, Nat. Commun. 5, 3301 (2014).
- D.E. Salt, I. Baxter, and B. Lahner, Annu. Rev. Plant Biol. 59, 709–733 (2008).
- A. Praetorius, A. Gundlach-Graham, E. Goldberg, W. Fabienke, J. Navratilova, A. Gondikas, R. Kaegi, D. Günther, T. Hofmann, and F. Von Der Kammer, Environ. Sci. Nano. 4, 307–314 (2017).
- O. Borovinskaya, S. Aulakh, and R. Markus, Tofw. Appilcation Note 1–3 (2019).
- M. von der Au, O. Borovinskaya, L. Flamigni, K. Kuhlmeier, C. Büchel, and B. Meermann, Algal Res. 49, 101964 (2020).
- L. Mueller, H. Traub, N. Jakubowski, D. Drescher, V.I. Baranov, and J. Kneipp, Anal. Bioanal. Chem. 406, 6963–6977 (2014).
- F. Piccapietra, C.G. Allue, L. Sigg, and R. Behra, Environ. Sci. Technol. 46, 7390–7397 (2012).
- F. Perreault, A. Oukarroum, S. P. Melegari, W.G. Matias, and R. Popovic, Chemosphere 87, 1388–1394 (2012).
- L.H.S. Jensen, L.M. Skjolding, A. Thit, S.N. Sørensen, C. Købler, K. Mølhave, and A. Baun, Environ. Toxicol. Chem. 36(6), 1503–1509 (2016).
- C. Brandenberger, M.J.D. Clift, D. Vanhecke, C. Mühlfeld, V. Stone, P. Gehr, and B. Rothen-Rutishauser, Part. Fibre Toxicol. 7, 15 (2010).
- ISO, International Organization for Standarization. ISO 8692, “Water Quality-Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae” (2012).
- J. Zhao, X. Cao, X. Liu, Z. Wang, C. Zhang, J.C. White, and B. Xing, Nanotoxicology 10(9), 1297–1305 (2016).
- F. Chen, Z. Xiao, L. Yue, J. Wang, Y. Feng, X. Zhu, Z. Wang, and B. Xing, Environ. Sci. Nano. 6, 1026–1042 (2019).
- S. Theiner, A. Schoeberl, S. Neumayer, and G. Koellensperger, J. Anal. At. Spectrom. 34, 1272–1278 (2019).
- S. Theiner, A. Schweikert, C. Haberler, A. Peyrl, and G. Koellensperger, Metallomics 12, 1246–1252 (2020).

Lyndsey Hendriks is an application scientist at TOFWerk AG, in Thun, Switzerland. She has a PhD from ETH Zurich.Direct correspondence to: lyndsey.hendriks@tofwerk.com

Lars Michael Skjolding has a PhD in nanoecotoxicology from the Technical University of Denmark. In 2018, he joined the EU project PATROLS (Physiologically Anchored Tool for Realistic nanomaterial hazard assessment) assessing physiological responses of algal and nanoparticle interaction.

Robert Thomas is the principal of Scientific Solutions, an educational consulting company that serves the training and writing needs of the trace element user community. He has worked in the field of atomic and mass spectroscopy for more than 45 years.

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
Atomic Perspectives: Highlights from Recent Columns
March 3rd 2025“Atomic Perspectives,” provides tutorials and updates on new analytical atomic spectroscopy techniques in a broad range of applications, including environmental analysis, food and beverage analysis, and space exploration, to name a few. Here, we present a compilation of some of the most popular columns.