Testing Electronic Device Components for RoHS/WEEE Compliance Using Microwave Digestion and ICP-OES
The combination of microwave sample preparation and ICP-OES is examined to meet the challenges of measuring a suite of heavy metals in a wide range of electronic components for RoHS/WEEE compliance.
With electronic devices being such an integral part of our everyday lives, the correct handling of their disposal is of great environmental importance. Many of the components within these devices can contain hazardous substances and, for that reason, the Restriction of Hazardous Substances (RoHS) regulations together with the Waste Electrical and Electronic Equipment (WEEE) directives were created in Europe to monitor a suite of heavy metals and organic compounds commonly found in device components. To comply with these directives, manufacturers of electronic devices must show that their products do not exceed the regulatory levels for these substances. The main analytical challenge in determining heavy metal levels in these materials is sample preparation, specifically how to prepare such diverse sample types without losing volatile elements, particularly mercury, during the digestion process. This study evaluates the combination of microwave sample preparation and inductively coupled plasma–optical emission spectrometry (ICP-OES) to meet the challenges of measuring a suite of heavy metals in a wide range of electronic components for RoHS/WEEE compliance.
Manufacturers and suppliers of electronic and electrical products in countries within the European Union (EU) must comply with Restriction of Hazardous Substances (RoHS) (1) and Waste from Electrical and Electronic Equipment (WEEE) regulations (2). In addition, companies outside of Europe that wish to sell their products in the EU must also comply with these directives. Typical products that currently fall under these regulations include
- large household appliances such as refrigerators, washers, stoves, air conditioners;
- small household appliances such as vacuum cleaners, hair dryers, and coffee makers;
- computing and communications equipment such as computers, printers, copiers, and phones;
- consumer electronics such as TVs, DVD players, stereos, and video cameras;
- lighting such as lamps, lighting fixtures, and light bulbs;
- power tools such as drills, saws, nail guns, sprayers, and trimmers;
- toys and sports equipment such as video games, electric trains, and treadmills;
- automatic dispensers such as vending machines and ATM machines.
Under these directives, the use and disposal of 10 hazardous metals or materials used in the manufacture of these products is restricted. The hazardous materials defined in the RoHS/WEEE regulations include
- Lead (Pb)
- Mercury (Hg)
- Cadmium (Cd)
- Hexavalent chromium (Cr[VI])
- Polybrominated biphenyls (PBB)
- Polybrominated diphenyl ether (PBDE)
- Bis(2-ethylhexyl) phthalate (DEHP)
- Butyl benzyl phthalate (BBP)
- Dibutyl phthalate (DBP)
- Diisobutyl phthalate (DIBP)
The maximum permitted concentrations for these regulated analytes are 1000 ppm (except for cadmium, which is limited to 100 ppm) by weight of homogeneous material. The term homogeneous means that the limits do not apply to the total weight of the final product, or even to an individual item in the product, but to any single component that could be mechanically separated out. For example, in a printed circuit board, this term would refer to the plastic substrate material, the metallic connecting wire, the plastic sheath covering a cable, or any other material used in the manufacturing process of the board.
In addition, with the increasing use of and reliance on electronics, manufacturers are continually developing new products with enhanced capabilities. As a result, the lifetime of electronics is becoming shorter as consumers more frequently upgrade to newer, more advanced models. The result is an increasing number of electronics being disposed of. Although electronics recycling programs have been implemented and are growing, the number of electronic products being discarded continues to rise.
Determination of RoHS Regulated Heavy Metals
The most widely used solid-sampling analytical technique for carrying out the rapid trace element testing of electrical equipment to meet the RoHS/WEEE requirements has traditionally been X-ray fluorescence (XRF) spectroscopy (3). However, the inherent problem with this technique is that the X-ray beam diameter is typically only a few hundred micrometers. If the component is extremely small, accurate quantitation could be problematic. In addition, if there is any elemental segregation in the electrical component, the composition could be different depending on where the X-ray beam strikes the sample. These potential limitations could mean that an item may pass or fail the RoHS/WEEE levels based on a measurement that is not truly representative of the entire component.
To get around many of these inherent limitations of XRF, the simplest and most efficient way to meet the RoHS directive for Pb, Hg, Cd, and Cr(VI) is to digest the samples using microwave digestion and determine the elemental concentrations with inductively coupled plasma–optical emission spectroscopy (ICP-OES). Although ICP-OES cannot distinguish different forms of elements, the total Cr concentration can be measured to determine if it is above or below the regulated level. If it is above the limit, the samples can be further prepared and analyzed by an elemental speciation technique to determine the concentration of Cr(VI) (4).
This study focuses on the analysis of a variety of sample materials present in a number of electronic components for compliance with the RoHS/WEEE directives using ICP-OES and, in particular, to optimize the sample preparation procedure.
Methodology
Since there are a wide variety of sample types that fall under the RoHS directive, it is impossible to evaluate all of them. For that reason, a representative selection of sample types was analyzed:
- plastics
- wire insulation
- solder
- wire
- circuit board
All materials were taken from discarded electronic products (with the exception of the solder, which was new). Given the diversity of possible sample types, microwave digestion provides the most flexibility and highest probability of complete digestion. While it is possible to optimize the microwave methodology for each sample type, the goal was to develop a single sample preparation method that would be applicable to many sample types to simplify the sample preparation process. Although a single method was not possible for this sample set, the preparation variations between sample types were minimized.
Table I shows details of the nine samples evaluated, which were collected from discarded electronic equipment (with the exception of the solder, which was purchased new). Each sample was cut into small pieces, and 0.2-g portions were used for analysis.
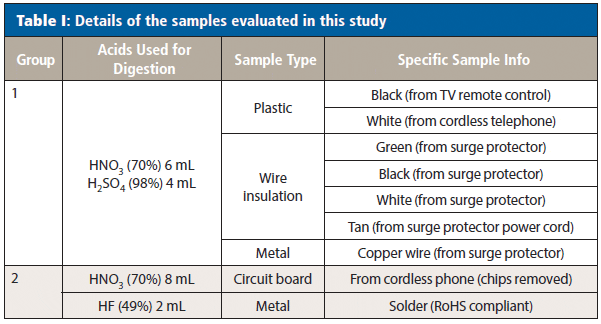
Sample Preparation
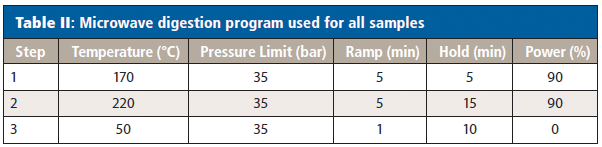
All samples were digested using a Titan MPS microwave digestion system (PerkinElmer Inc.) (5). First, 0.2 g of sample was added to each digestion vessel, followed by the appropriate acids (trace metal grade) as shown in Table III, with the nitric acid added first to each. The vessels were left open for about 10 min to allow for any predigestion that may occur before being sealed and placed in the microwave digestion system. Table II shows the microwave digestion program used for all samples; only the acids differed between the sample types.
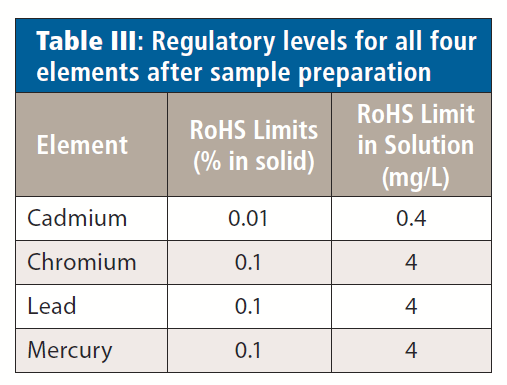
After digestion, the samples were diluted to 50 mL with 5% HCl (v/v) to complex the mercury. The exception was the solder, which was diluted with deionized (DI) water. Using this sample preparation procedure, the regulatory levels for the elements in solution are shown in Table III.
All quantitative measurements were made against calibration curves prepared in a mixture of 10% nitric acid (v/v), 5% sulfuric acid (v/v), and 1% hydrochloric acid (v/v) using 0.5 and 1 ppm standards, with 0.5 ppm yttrium used as an internal standard. To assess accuracy, predigestion spikes at the regulatory levels and 10× below were added to the digestion vessels before adding the acids.
Instrumental Conditions
All analyses were carried out using an Avio 200 ICP-OES system (PerkinElmer Inc.) operating in the axial mode. The instrument, which has been described in the public domain, is a compact, bench-mounted system designed to provide approximately 50% lower argon consumption (6).
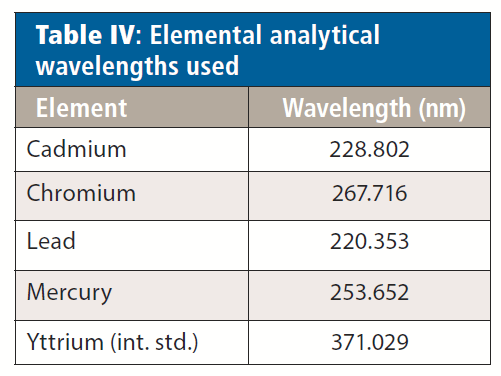
The elements and analytical wavelengths used for analysis are listed in Table IV, and the instrumental parameters appear in Table V. Yttrium was added to all standards and samples at 0.5 ppm. The rinse solution contained 5% nitric acid (v/v) and 2% hydrochloric acid (v/v), with the HCl being present to aid in mercury washout and memory effects.
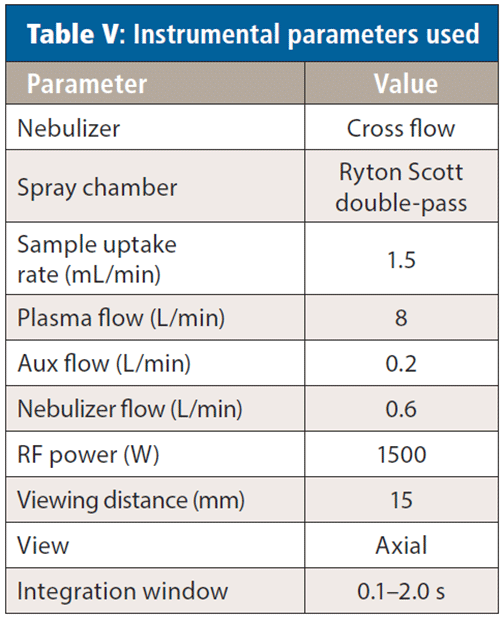
Because the compliance limits for RoHS/WEEE are well within the instrument's detection capability, a short auto integration time window was used, increasing analytical speed and sample throughput without sacrificing accuracy. It should also be noted that all analyses were performed using a plasma gas flow of 8 L/min.
Results and Discussion
Although the objective was to use a single acid mixture and one microwave digestion program to determine the four heavy metals in the samples, it necessitated two different acid mixtures to effectively digest all samples. However, the same digestion procedure was used for both acid mixtures. Because of the diverse nature of the samples, sulfuric acid was found to be very effective because it lowers the vapor pressure in the vessels, allowing higher temperatures to be attained and thus significantly increasing the efficiency of the nitric acid. However, the sulfuric–nitric acid mixture did not completely dissolve the circuit board or solder, most likely because of the presence of silica. As a result, for these samples, hydrofluoric acid was found to be effective when mixed with nitric acid.
In all cases, a significant amount of gas was generated during the digestion. Most of the dissolved gas was expelled during transfer to the autosampler tubes and dilution. However, it is recommended that the samples be allowed to sit in open containers for 30 min before analysis to completely degas. Dissolved gas present in the samples results in high relative standard deviations during the analysis.
Although these conditions were found to be effective for all the samples in this study, it cannot be guaranteed that they will yield complete digestions for all possible sample types covered under the RoHS/WEEE directives, but they can serve as a starting point. Regardless of the required acid combinations, closed-vessel digestion is strongly suggested to prevent loss of Hg, a volatile element.
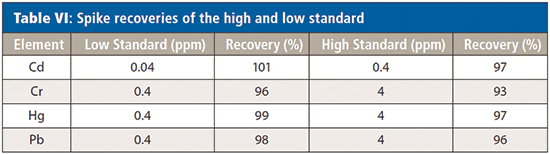
To establish the accuracy of the calibration, check standards at the RoHS regulated level, together with standards 10 times below the RoHS levels (in solution) were analyzed directly after the calibration curves. Table VI shows the recoveries of both standards, which indicate good accuracy at both low and high levels.
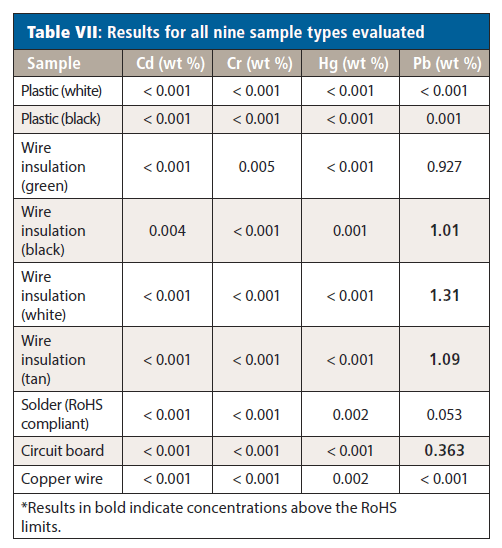
With the ability to accurately measure both low and high concentrations, the samples were analyzed; the results are shown in Table VII. For Cd, Cr, and Hg, all samples were below the RoHS/WEEE limits. However, the Pb levels varied significantly between samples. Both plastics and the copper wire had Pb present below the regulated levels. Although there is Pb present in the solder, it is also below the RoHS level, confirming that the solder is RoHS compliant. However, Pb was above the regulated level in all of the wire insulations and the circuit board; repeated analyses of multiple samples of all the wire insulations and circuit board yielded equivalent results. The high Pb values from the circuit board most likely result from the use of non-RoHS/WEEE-compliant solder.
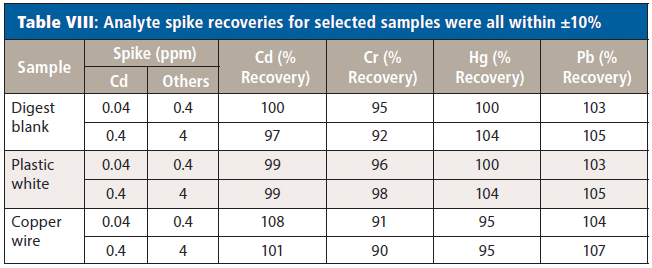
To assess the accuracy of the methodology and confirm that the sample preparation process does not affect recoveries, predigestion spikes were evaluated. For selected samples, spikes were added to the digestion vessels after the addition of sample, but before the acids were added. Spikes were made at two levels: 10 times below and at the RoHS levels (in solution). The Cd was spiked at concentrations 10 times lower than the other elements, because of the RoHS regulations being 10x lower for Cd than the other elements. All recoveries are within 10% of the spiked values (as shown in Table VIII), indicating that no significant contamination or element loss occurs during the digestion process. The accuracy of the methodology is also validated by the accuracy of the spike recoveries.
Conclusion
This study has demonstrated the ability of microwave digestion coupled with ICP-OES to rapidly and accurately measure elements in a wide variety of sample types covered by RoHS/WEEE directives. With the optimum choice of acids, a variety of different sample types can be digested using a single microwave program, minimizing the number of different sample preparation steps. In addition, predigestion spike recoveries prove that elements are not lost during the digestion process and that as long as well-recognized sample preparation cleanliness procedures are followed, contamination is not introduced, and as a result, good data quality objectives can be achieved.
References
(1) Restriction of Hazardous Substances (RoHS) Directive 2002/95/EC, http://www.rohsguide.com/.
(2) European Union Waste Electrical & Electronic Equipment (WEEE) Directive 2002/96/EC http://ec.europa.eu/environment/waste/weee/index_en.htm.
(3) J.E. Martin et.al., Spectroscopy 25(4), 40–45 (2010).
(4) H. Ernstberger and K. Neubauer, "Chromium Speciation in Drinking Water by LC-ICP-MS," PerkinElmer Inc. Application Note, https://www.perkinelmer.com/lab-solutions/resources/docs/APP_Altus-UPLC-NexION-350-ICP-MS-Cr-Speciation-in-Drinking-Water-012309_01.pdf.
(5) "Titan Microwave Digestion Application Notebook," http://www.perkinelmer.com/lab-solutions/resources/docs/APP_Titan-MPS-Applications-Notebook_010773B_01.pdf.
(6) Avio 200 ICP Optical Emission Spectrometer, PerkinElmer Inc., Product Specifications, http://pesystems.cz/wp-content/uploads/2016/10/Avio-200-ICP-OES-Specifications-012884_01.pdf.
K. Neubauer is with PerkinElmer Inc., in Shelton, Connecticut. Direct correspondence to: Kenneth.Neubauer@perkinelmer.com

Geographical Traceability of Millet by Mid-Infrared Spectroscopy and Feature Extraction
February 13th 2025The study developed an effective mid-infrared spectroscopic identification model, combining principal component analysis (PCA) and support vector machine (SVM), to accurately determine the geographical origin of five types of millet with a recognition accuracy of up to 99.2% for the training set and 98.3% for the prediction set.
Authenticity Identification of Panax notoginseng by Terahertz Spectroscopy Combined with LS-SVM
In this article, it is explored whether THz-TDS combined with LS-SVM can be used to effectively identify the authenticity of Panax notoginseng, a traditional Chinese medicine.