The Application of Atomic Spectroscopy Techniques in the Recovery of Critical Raw Materials from Industrial Waste Streams, Part II
This month’s column is Part 2 of a contribution from my daughter Glenna, who recently completed her PhD studies in Environmental Science from the University of Copenhagen in Denmark. Her article explores the current landscape of global critical raw materials (CRM) trends in research and the applications of atomic spectroscopy (AS), including inductively coupled plasma–mass spectrometry (ICP-MS), inductively coupled plasma–optical emission spectrometry (ICP-OES), and X-ray analytical techniques in their identification of diverse industrial and environmental media, which have been essential in method validation and quantification of CRMs in complex matrices presenting high risks of interference. Some important examples to be presented include rare earth elements (REEs) in water leaching purification (WLP) residues that co-occur with radioactive materials; REEs and other metals in acid mine drainage (AMD) environments; REEs in coal combustion (fly ash) residues; arsenic (As) from groundwater treatment sediment; and platinum-group elements (PGEs) from sewage sludge. The article also classifies the different techniques in use at each stage of the CRM recovery train, investigates present challenges to each analytical method, and discusses the problem-solving tools used.
- Rob Thomas, Column Editor
Part 1 of this article focused on the overview of critical raw materials (CRMs), the global context for sustainable sourcing of CRMs, and the recent trends in the application of atomic spectroscopy (AS) techniques in CRM recovery-related research. Here, in Part 2, the challenges faced in CRM determination by AS techniques are explained and various solutions are presented, with a focus on inductively coupled plasma–mass spectrometry (ICP-MS) and inductively coupled plasma–optical emission spectrometry (ICP-OES) for measuring rare earth elements (REEs), platinum group elements (PGEs), and arsenic (As).
The challenges encountered in CRM analysis by AS are based on many factors, ranging from the chosen analytical technique and sample preparation to instrument optimization and the analytes themselves. In general, the problems tend to fall into three core categories: spectral interference; non-spectral interference (matrix effects); and quality control.
Spectral Interference
Spectral interference refers to the overlap of the spectral line from other elements in the sample matrix with that of the analytes of interest. In the context of ICP-MS, which is most often used for REE and PGE analysis, this occurs when element isotopes share the same mass-to-charge (m/z) ratio (isobaric interference), or when two or more isotopes combine to have the same m/z as another element’s isotope (polyatomic interference).
For the analysis of REEs by ICP-MS, often preferred over ICP-OES because of lower detection limits, there are certain spectral interferences that cause problems for the quantification of individual REEs as well as for the full suite. REEs form strong metal oxide bonds (MO+) and metal hydride bonds (MH+) (1). The MO+ ions from lower mass REEs will overlap in their m/z ratio with the isotopes of higher mass REEs (Tb-Lu). A list of the common REE interferences from MO+ ions is shown in Table I.
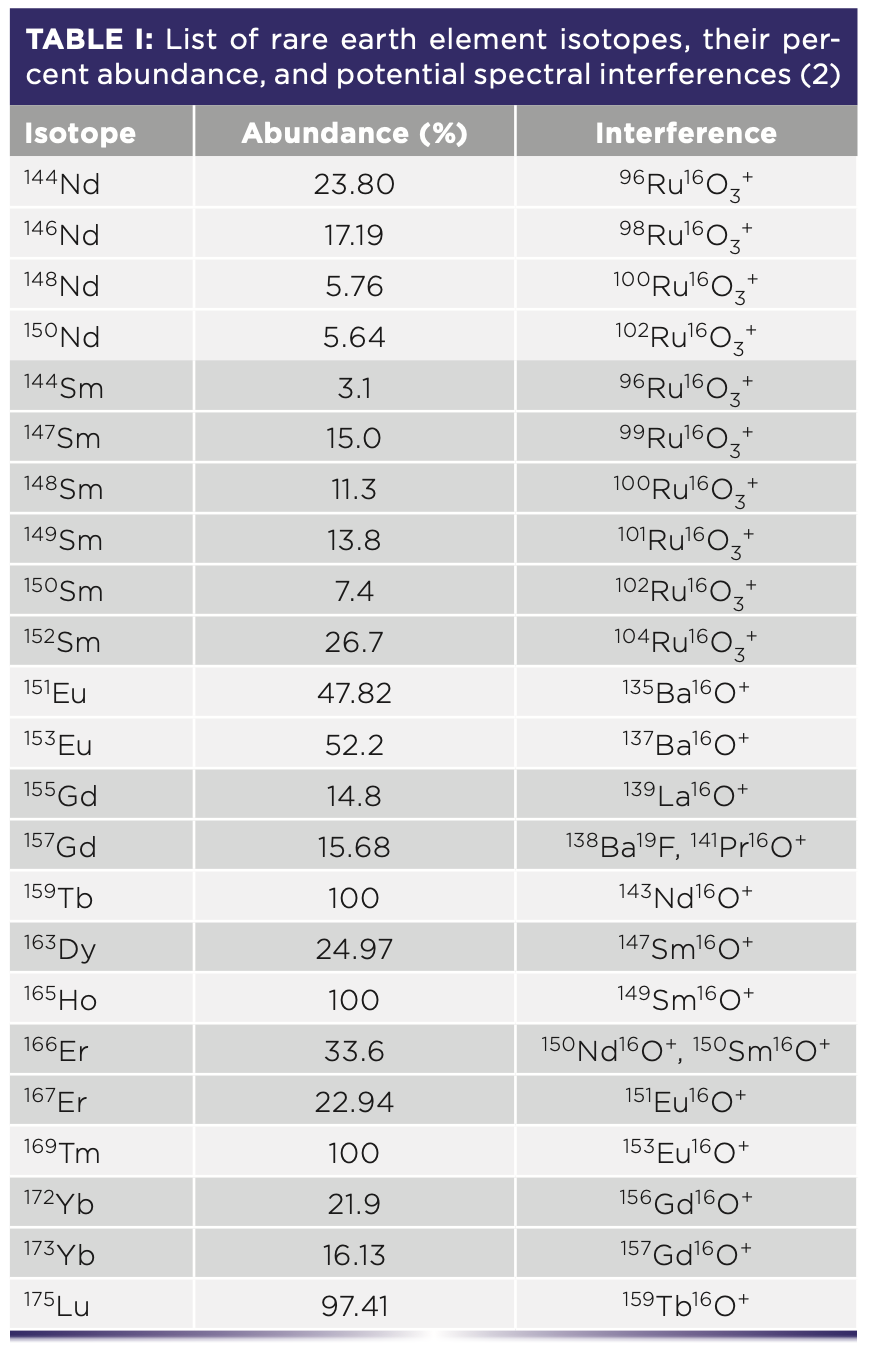
For the most reliable determination of the full suite of REEs in a high REE-oxide matrix, single quadrupole or triple/multi quadrupole ICP-MS (ICP–QQQ) is used. The concentrations of lower mass REEs (La, Ce, Nd, Sm, Gd, Tb, and Lu) can reliably be determined by using the O2 mass-shift mode, whereas the NH3 cell gas mode is better for higher mass REEs (Pr, Eu, Dy, Ho, Er, Tm, and Yb) (1). Interferences from high REE-oxide purity samples is a relevant factor for a variety of industrial waste products (Table II).
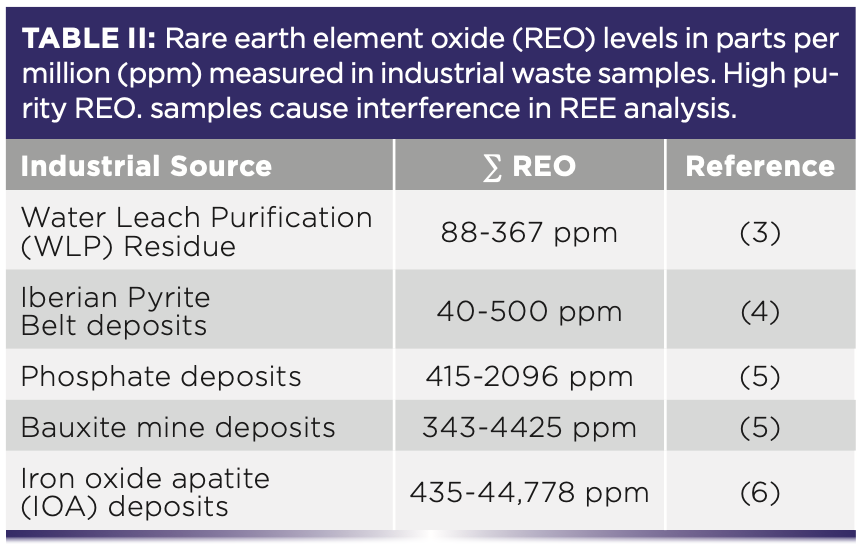
Likewise, the MH+ ions of La and Ba interfere with Ce and La analysis, respectively (1). Changing the cell gas mode allows for the selection of alternative isotopes for Ce and La if the analysis is isolated. As an alternative to changing the cell gas mode when ICP–QQQ is unavailable, isotope dilution mass spectrometry (IDMS) can be applied—a process that adds enriched isotopes to the samples allowing for various MO+ and MH+ interferences to be resolved (7). IDMS is not a fix-all, as it does still result in some interferences between enriched REE isotopes and the REE analytes (7). In cases such as this, methods of matrix separation are often employed where analytes are isolated from unwanted elements in the matrix, such as with ion-exchange (IX) where REEs are eluted out in order of their atomic radii (7). Further discussion is given to matrix separation in the following section.
The major issues of spectral interference for PGEs include the overlap of HfO+ ions with Pt, of ZrO+, YO+, SrO+, MoO+, and Cd isotopes with Pd, and of Pb, ArCu+, RbO+, and SrO+ isotopes with 103Rh (8). Additionally, hydride, argide, and chloride interferences are problematic for individual PGEs (Table III).
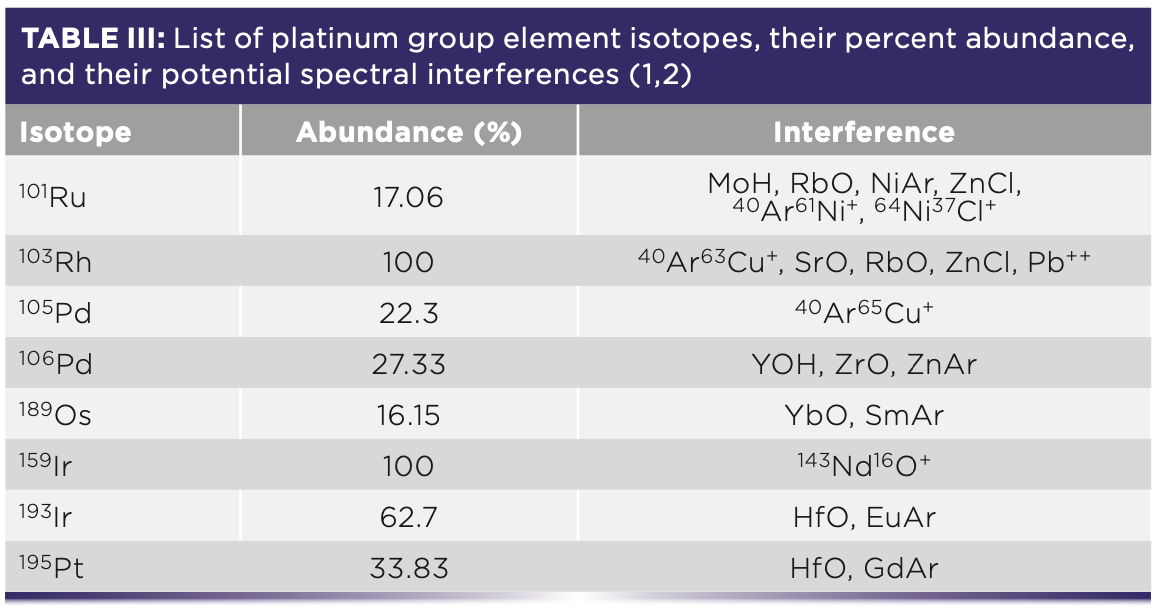
Methods to correct for or avoid these spectral interferences include applying mathematical corrections, separating analytes from the matrix (see next section), and using high-resolution (HR)–ICP-MS (1). Of the three, HR–ICP-MS is the most reliable; however, certain mass resolutions, such as 103Rh from 87Sr16O+ (M/ΔM = 102,900) and 105Pd from 89Y16O+ (M/ΔM = 27,600), are still unable to be resolved (1). The overlap of polyatomic ions occurring in the quadrupole is typically handled using helium as the reaction or collision gas; however, this does not remove interference from non-polyatomic ions (such as 206Pb++ on Rh) (1).
Although graphite furnace atomic absorption spectrometry (GFAAS) is a robust and reliable AS technique for As, ICP-MS might be preferred in these cases because it allows for the determination of multiple elements in one sample run. There are a number of possible spectral interferences occurring for As analysis by ICP-MS (Table IV).
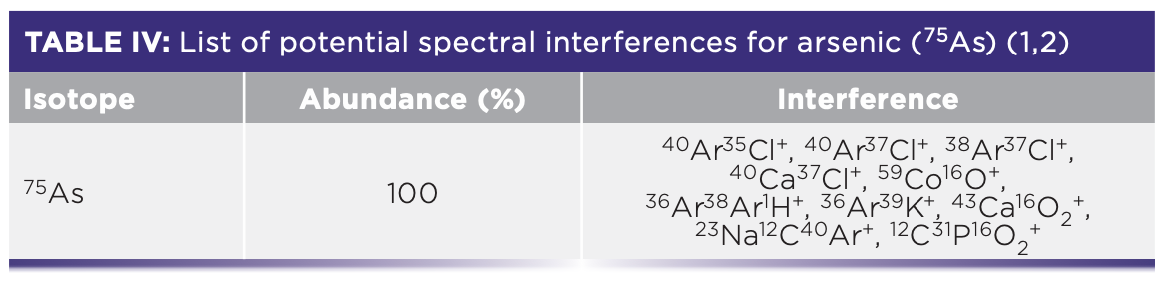
Because of the overlap of argon chloride ions, helium (He) or hydrogen is often used as the collision/reaction gas in triple or multi quadrupole ICP-MS (9). Additionally, a number of issues arise in As determination by ICP-MS from doubly charged ions (++), which appear at half of their true mass and overlap with singly charged As ion with the same overall m/z (1). This occurs with the REEs 150Sm++ and 150Nd++ and can either be corrected using mathematical correction equations or resolved by using ICP–QQQ in MS/MS reaction mode and measuring AsO+ at m/z = 91 (1).
Non-Spectral Interference-Matrix Effects
Non-spectral interference describes the interference that might occur on sample transport and on the analyte’s signal response (suppression or enhancement) because of the sample matrix. These matrix effects appear to be less of an issue for PGEs than for REEs in ICP-MS analysis (10,11). Barium is the most common culprit for matrix effects in REE determination. It is found in high concentrations in natural waters and industrial wastewaters causing interference for Eu+ from BaO+ and on La+ from BaH+ (12–14). Arsenic might experience non-spectral interference in high carbon and sodium-rich sample matrices if the calibration standards do not match the contents, which occurs in both ICP-MS and ICP-OES analysis (15). Non-spectral interference on PGEs has been observed in high Fe and Al and in some cases Si and Cu-matrices when analyzed by ICP-OES (16). In general, dissolved salts, organics, or total dissolved solids (TDS) cause the most serious matrix effects resulting in a lowered ionization efficiency, signal suppression or enhancement and/or instrumental drift for ICP-MS and ICP-OES (15,17).
There are various methods available for avoiding matrix effects. These include 1) sample dilution, if the analytes are in a high enough concentration in solution; 2) matching matrix concentrations in the calibration solutions; 3) the use of internal standards (Ru, Re, and Ca for REE [13,14], Se for As [18], and Ru, Ir, In, and Au for PGEs [19]); 4) increasing plasma temperature; and 5) lowering nebulizer flow (15). Analytes can also be separated from their matrix and preconcentrated in the sample. This has been a well-explored alternative for improving REE and PGE determination by ICP-MS. Techniques here can be classified as liquid–liquid extraction (LLE), sorbent-based extraction (SBE) including solid-phase extraction (SPE), and chelating and ion exchange (IX) resins (19–21). Table V shows a selection of matrix separation methods and materials used for REEs and PGEs from industrially-sourced samples followed by ICP-MS, ICP-OES, or other AS analytical techniques.
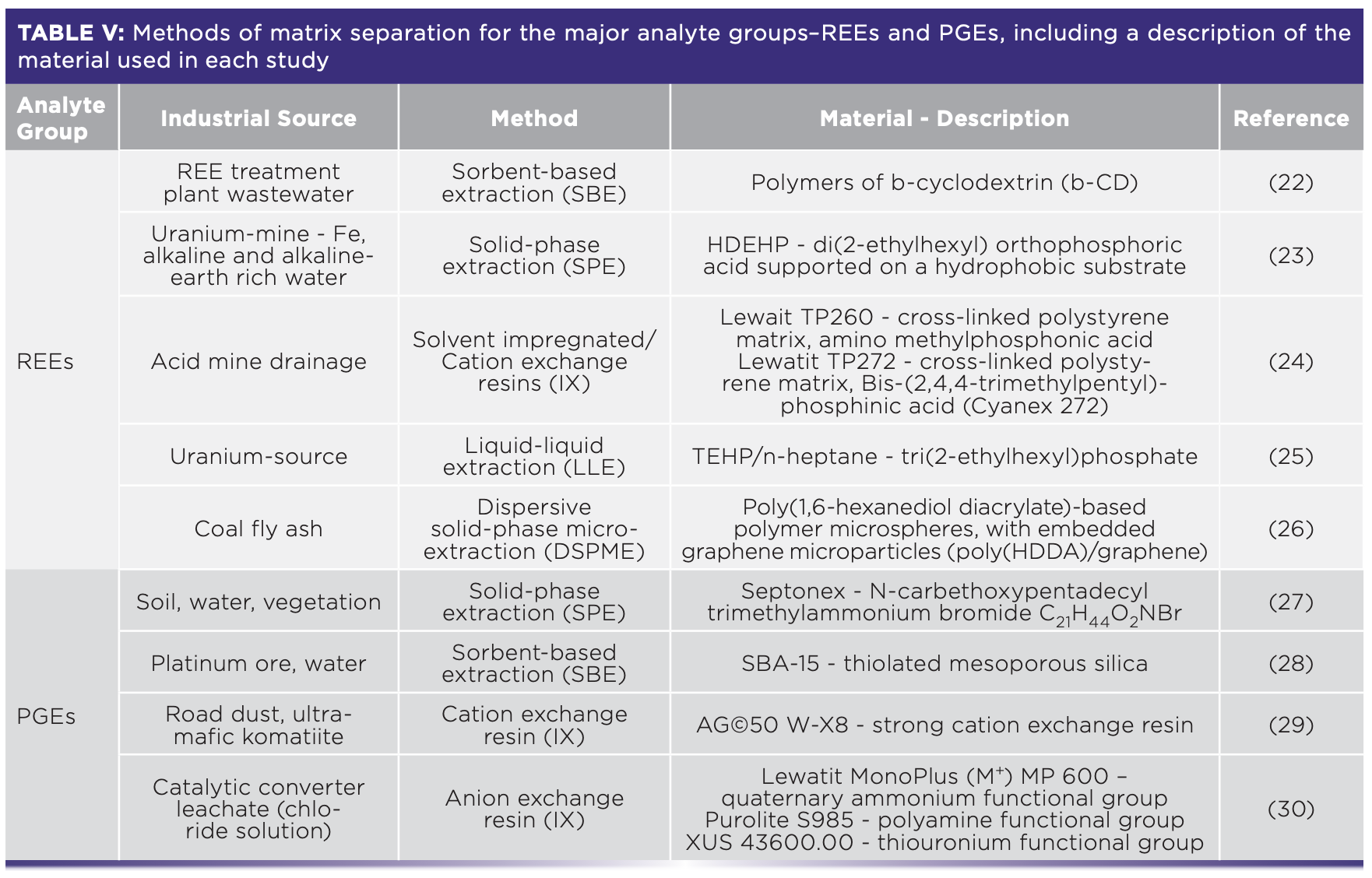
The removal of As from drinking water, natural water, and wastewater as a preconcentration approach is mostly done with anion exchange and hybrid chelating resins, which perform relatively well depending on pH conditions (31). However, the main challenge resides in separating As from the often Fe-rich material it has co-precipitated within the industrial treatment process, rather than the sample requiring matrix separation for analytical accuracy.
Quality Control
This third category of quality control points to the analytical challenges faced with a lack of suitable certified or standard reference materials (SRMs) for a given sample and the critical raw materials (CRMs) of interest. SRMs are used as a form of quality control in AS techniques, and, in order to evaluate and validate test procedures, the material matrix should match as closely as possible that of the sample. REE determination in crushed stone, mine water, and coal ash samples by ICP-MS and ICP-OES have based their recoveries on reference materials from basalt and granite (12), standard reference water (32), and marine shale and ore (33), respectively—none of which are ideal matches. Industrial wastewater, sludge, and residues neither resemble soils and sediments in matrix composition, although there are other reference materials used in the absence of a better alternative (34–36). For PGEs, most reference materials available are from ores and car exhaust catalysts with very few options for soil, sediment, plant, and road dust materials (8). SRMs used for arsenic determination in industrial samples tend to originate from contaminated soil and contaminated water-based matrices and have shown recoveries within a +/- 5% margin (37). What is needed imminently is a greater availability of standard reference materials with certifiable concentrations of critical raw materials in a variety of environmental and industrial sample types including rocks, sediments, soils, and water, along with sludges and residues.
Future Perspectives
In the coming years, critical raw materials (CRM) lists will continue to adapt as new geopolitical, socioeconomic and geological factors are taken into consideration. Countries are already beginning to invest in research and innovation to explore and develop local primary and secondary sources of CRMs. In August 2023, the U.S. Department of Energy announced that up to $30 million USD financed by the Bipartisan Infrastructure Law would be available for investment in projects that lower the cost of on-shore production of REEs and other CRMs specifically from coal-based resources (38). This is in addition to an already $41 million USD invested in projects geared towards CRM resource identification, extraction, production, and processing from secondary sources like mining waste (38). Ongoing and future research will continue to rely on AS techniques for the reliable analysis of CRMs at various stages of their identification and recovery from secondary sources. Therefore, it is of utmost importance that industry continues to develop method optimization and quality control procedures that can support the green and circular transition of CRM-reliant industries.
References
(1) Handbook of ICP-QQQ Applications Using the Agilent 880 and 8900; 5th edition. (accessed 2023-03-12).
(2) May, T.W.; Wiedmeyer, R.H.A Table of Polyatomic Interferences in ICP-MS. At. Spectrosc. 1998, 19 (5), 150–155. DOI: 10.46770/AS.1998.05.002
(3) Amidi, A.; Razif, S. A. M.; Jabit, N. A.; Ariffin, K. S. 2022. Characterization of Rare Earth Elements (REE) from Industrial REE Waste Resources. Mater. Today: Proc. 2022, 66, 3140–3143. DOI: 10.1016/j.matpr.2022.07.464
(4) Pérez-López, R.; Delgado, J.; Nieto, J. M. ; Márquez-García, B., Rare Earth Element Geochemistry of Sulphide Weathering in the São Domingos Mine Area (Iberian Pyrite Belt): A Proxy for Fluid–Rock Interaction and Ancient Mining Pollution. Chem. Geol. 2010, 276 (1–2), 29–40. DOI: 10.1016/j.chemgeo.2010.05.018
(5) Pyrgaki, K.; Gemeni, V.; Karkalis, C.; Koukouzas, N.; Koutsovitis, P.; Petrounias, P., 2021. Geochemical Occurrence of Rare Earth Elements in Mining Waste and Mine Water: A Review. Minerals 2021, 11 (8), 860. DOI: 10.3390/min11080860
(6) Taylor, R. D.; Shah, A. K.; Walsh, G. J.; Taylor, C. D., Geochemistry and Geophysics of Iron Oxide-Apatite Deposits and Associated Waste Piles with Implications for Potential Rare Earth Element Resources from Ore and Historical Mine Waste in the Eastern Adirondack Highlands, New York, USA. Econ. Geol. 2019, 114 (8), 1569–1598. DOI: 10.5382/econgeo.4689
(7) Bradley, V.. C.; Manard, B. T.; Roach, B. D.; Metzger, S. C.; Rogers, K. T.; Ticknor, B. W.; Wysor, S. K.;Brockman, J. D.; Hexel, C. R. Rare Earth Element Determination in Uranium Ore Concentrates Using Online and Offline Chromatography Coupled to ICP-MS. Minerals 2020, 10 (1), 55. DOI: 10.3390/min10010055
(8) Djingova, R.; Heidenreich, H.: Kovacheva, P. ; Markert, B. On the Determination of Platinum Group Elements in Environmental Materials by Inductively Coupled Plasma Mass Spectrometry and Microwave Digestion. Anal. Chim. Acta 2003, 489 (2), 245–251. DOI: 10.1016/S0003-2670(03)00716-5
(9) Nawrocka, A., Durkalec, M., Michalski, M. and Posyniak, A.Simple and Reliable Determination of Total Arsenic and its Species in Seafood by ICP-MS and HPLC-ICP-MS. Food Chem. 2022, 379, 132045. DOI: 10.1016/j.foodchem.2022.132045
(10) Bencs, L.; Ravindra, K.; Van Grieken, R. Methods for the Determination of Platinum Group Elements Originating from the Abrasion of Automotive Ctalytic Converters. Spectrochim. Acta Part B 2033, 58 (10), 1723–1755. DOI: 10.1016/S0584-8547(03)00162-9
(11) Kokh, M. A.; Luais, B.; Truche, L.; Boiron, M. C.; Peiffert, C.; Schumacher, A. Quantitative Measurement of Rare Earth Elements in Brines: Isolation from the Charged Matrix Versus Direct LA‐ICP‐MS Measurements–A Comparative Study. Geostand. Geoan. Res. 2021, 45 (2), 341–358. DOI: 10.1111/ggr.12376
(12) Rojano, W. J. S.; dos Anjos, T.; Duyck, C. B.;Saint’Pierre, T. D. Determination of Rare Earth Elements in Environmental Samples with High Concentrations of Barium by Quadrupole Inductively Coupled Plasma Mass Spectrometry. Microchem. J. 2019, 149, 104026. DOI: 10.1016/j.microc.2019.104026
(13) Merten, D.; Büchel, G., 2004. Determination of Rare Earth Elements in Acid Mine Drainage by Inductively Coupled Plasma Mass Spectrometry. Microchim. Acta 2004, 148, 163–170. DOI: 10.1007/s00604-004-0260-0
(14) Sindern, S. Analysis of Rare Earth Elements in Rock and Mineral Samples by ICP-MS and LA-ICP-MS. Physical Sciences Reviews 2017, 2 (2), 20160066. DOI: 10.1515/psr-2016-0066
(15) Pruszkowski, E. Interferences in ICP-MS: Do We Still Have to Worry About Them? PerkinElmer, Inc. 2021.
(16) Kim, J.; Anawati, J.; Azimi, G., Matrix Complexity Effect on Platinum Group Metals Analysis Using Inductively Coupled Plasma Optical Emission Spectrometry. J. Anal. At. Spectrom. 2018, 33 (8), 1310–1321. DOI: 10.1039/C8JA00158H
(17) Kokh, M. A.; Luais, B.; Truche, L.; Boiron, M. C.; Peiffert, C.; Schumacher, A. Quantitative Measurement of Rare Earth Elements in Brines: Isolation from the Charged Matrix Versus Direct LA‐ICP‐MS Measurements–A Comparative Study. Geostand. Geoanal. Res. 2021, 45 (2), 341–358. DOI: 10.1111/ggr.12376
(18) Kaňa, A.; Klimšová, Z.; Mestek, O., Internal Standardization for Arsenic and its Species Determination Using Inductively Coupled Plasma Mass Spectrometry. Talanta 2019, 192, 86–92. DOI: 10.1016/j.talanta.2018.09.038
(19) Balcerzak, M. Methods for the Determination of Platinum Group Elements in Environmental and Biological Materials: A Review. Crit. Rev. Anal. Chem. 2011, 41 (3), 214–235. DOI: 10.1080/10408347.2011.588922
(20) Komendova, R. Recent Advances in the Preconcentration and Determination of Platinum Group Metals in Environmental and Biological Samples. TrAC, Trends Anal. Chem. 2020, 122, 115708. DOI: 10.1016/j.trac.2019.115708
(21) Zawisza, B.; Pytlakowska, K.; Feist, B.; Polowniak, M.; Kita, A.; Sitko, R. Determination of Rare Earth Elements by Spectroscopic Techniques: A Review. J. Anal. At. Spectrom. 2011, 26 (12), 2373–2390. DOI: 10.1039/C1JA10140D
(22) Nkinahamira, F.; Alsbaiee, A.: Zeng, Q.; Li, Y., Zhang, Y.; Feng, M.; Yu, C. P.; Sun, Q., Selective and Fast Recovery of Rare Earth Elements from Industrial Wastewater by Porous β-cyclodextrin and Magnetic β-cyclodextrin olymers. Water Res. 2020, 181, 115857. DOI: 10.1016/j.watres.2020.115857
(23) Hernandez, G. C.; Cabezas, A. Q.; Diaz, M. F. Preconcentration and Determination of Rare-Earth Elements in Iron-Rich Water Samples by Extraction Chromatography and Plasma Source Mass Spectrometry (ICP-MS). Talanta 2005, 68, 47–53. DOI: 10.1016/j.talanta.2005.04.046
(24) Hermassi, M.; Granados, M.; Valderrama, C.; Ayora, C.; Cortina, J. L. Recovery of Rare Earth Elements from Aacidic Mine Waters by Integration of a Selective Chelating Ion-Exchanger and a Solvent Impregnated Resin. J. Environ. Chem. Eng. 2021, 9 (5), 105906. DOI: 10.1016/j.jece.2021.105906
(25) Baghaliannejad, R.; Aghahoseini, M.; Amini, M. K. Determination of Rare Earth Elements in Uranium Materials by ICP-MS and ICP-OES After Matrix Separation by Solvent Extraction with TEHP. Talanta 2021, 222, 121509. DOI: 10.1016/j.talanta.2020.121509
(26) Slavković-Beškoski, L.; Ignjatović, L.; Bolognesi, G.; Maksin, D.; Savić, A.; Vladisavljević, G.; Onjia, A., 2022. Dispersive Solid–Liquid Microextraction Based on the Poly (HDDA)/Graphene Sorbent Followed by ICP-MS for the Determination of Rare Earth Elements in Coal Fly Ash Leachate. Metals 2022, 12 (5), 791. DOI: 10.3390/met12050791
(27) Komendova, R.;Nevrla, J.; Kuta, J.; Sommer, L. Innovative Preconcentration Technique on Polymer Sorbent for Simultaneous Determination of Platinum Group Metals in the Waters and Lichen Hypogymnia Physodes. FEB-Fresenius EnvironmentalBulletin 2016, 25, 5172–5179. http://www.prt-parlar.de/download_feb_2016/
(28) Barczak, M.; Dobrzyńska, J.; Oszust, M.; Skwarek, E.; Ostrowski, J.; Zięba, E; Borowski, P.; Dobrowolski, R. Synthesis and Application of Thiolated Mesoporous Silicas for Sorption, Peconcentration and Determination of Platinum. Mater. Chem. Phys. 2016, 181, 126–135. DOI: 10.1016/j.matchemphys.2016.06.042
(29) Mitra, A.; Sen, I. S.; Walkner, C.; Meisel, T. C. Simultaneous Determination of Platinum Group Elements and Rhenium Mass Fractions in Road Dust Samples Using Isotope Dilution Inductively Coupled Plasma-Tandem Mass Spectrometry After Cation Exchange Separation. Spectrochim. Acta, Part B 2021, 177, 106052. DOI: 10.1016/j.sab.2020.106052
(30) Nikoloski, A. N.,; Ang, K. L.; Li, D. 2015. Recovery of Platinum, Palladium and Rhodium from Acidic Chloride Leach Solution Using Ion Exchange Resins. Hydrometallurgy 2015, 152, 20–32. DOI: 10.1016/j.hydromet.2014.12.006
(31) Issa, N. B.:Rajaković-Ognjanović, V.. N.; Marinković, A. D.; Rajaković, L. V., Separation and Determination of Arsenic Species in Water by Selective Exchange and Hybrid Resins. Anal. Chim. Acta 2011, 706 (1), 191–198. DOI: 10.1016/j.aca.2011.08.015
(32) Verplanck P. L.; Antweiler, R. C: Nordstrom, D. K.;Taylor H. E. Standard Reference Water Samples for Rare Earth Element Determinations. Appl. Geochem. 2001, 16, 231–244. DOI: 10.1016/S0883-2927(00)00030-5
(33) Thompson, R. L.; Bank, T.; Roth, E.; Granite, E. Resolution of Rare Earth Element Interferences in Fossil Energy By-Product Samples Using Sector-Field ICP-MS. Fuel 2016, 185, 94–101. DOI: 10.1016/j.fuel.2016.07.093
(34) Kramer, K. J.; de Haan, E. P.; van het Groenewoud, H.; Dorten, W.; Kramer, G. N.; Muntau, H. ; Quevauviller, P. Certified Reference Materials for the Quality Control of Rare Earth Element Determinations in the Environment. TrAC, Trends Anal. Chem. 2002, 21 (11), 762–773. DOI: 10.1016/S0165-9936(02)01101-9
(35) International Atomic Energy Agency. Certified Reference Material Certificate IAEA-158A Mass Fractions of Trace Elements in Marine Sediment Sample. 2008.
(36) Canadian Certified Reference Materials Project. Certificate of Analysis., TILL-1, TILL-2, TILL-3, TILL-4 Geochemical Soil and Till Reference Materials. 1995.
(37) Ahmad, W.; Alharthy, R. D.; Zubair, M.; Ahmed, M.; Hameed, A.; Rafique, S. Toxic and Heavy Metals Contamination Assessment in Soil and Water to Evaluate Human Health Risk. Sci. Rep. 2021, 11 (1), 17006. DOI: 10.1038/s41598-021-94616-4
(38) National Energy Technology Laboratory. “Biden-Harris Administration Announces $30 Million to Build Up Domestic Supply Chain for Critical Minerals. August 25, 2023 (accessed 2024-03-26). https://netl.doe.gov/node/12816
Glenna Thomas is an environmental research scientist and science illustrator who recently completed her PhD in Environmental Science at the University of Copenhagen in Denmark. Her research focuses on sustainable remediation techniques for soil and water contamination as well as recovery technology for critical materials. She has a background in geology, soil science and pedology and specializes in analytical techniques for the measurement of rare earth elements and heavy metals. ●

Robert Thomas is the principal scientist of Scientific Solutions, a consulting company that serves the educational needs of the trace element user community. He has worked in the field of atomic and mass spectroscopy for more than 50 years, including 24 years for a manufacturer of atomic spectroscopic instrumentation. Rob also serves on the editorial advisory board of Technology Networks and recently accepted a position of Assistant Adjunct Professor at the University of North Dakota. He has written over 100 technical publications, including a 15-part tutorial series on ICP-MS, in addition to authoring 6 textbooks on the fundamental principles and applications of ICP-MS. His most recent book published in October 2023 is entitled “Practical Guide to ICP-MS and Other Atomic Spectroscopy Techniques: A Tutorial for Beginners.” Rob has an advanced degree in analytical chemistry from the University of Wales, UK, and is also a Fellow of the Royal Society of Chemistry (FRSC) and a Chartered Chemist (CChem). ●


Microplastics Widespread on Catalan Beaches, Study Finds
March 28th 2025In a recent study published in Marine Pollution Bulletin, a team of researchers from several Spain and Portugal universities and institutions (Rovira i Virgili University, Universitat de Barcelona, University of Porto, and Institut d'Investigació Sanitaria Pere Virgili (IISPV) assessed microplastic (MP) contamination along the Mediterranean coastline.