Using Qualifier Ions to Validate Multielement ICP-MS Data in Complex Samples
Guest authors Steve Wilbur and Ed McCurdy discuss the role of qualifier ions in ICP-MS research in this month's installment of "Atomic Perspectives."

Inductively coupled plasma–mass spectrometry (ICP-MS) spectra are simple. This is both an advantage and a disadvantage. In the advantage category, they are easy to interpret. On the disadvantage side, this simplicity means that when interferences are present, there might not be enough additional information available to compensate. In general, an ICP-MS spectrum consists of a mass/intensity value for each isotope present in the sample. There are only about 240 naturally occurring isotopes of the 100 or so naturally occurring elements, so on average, each element has more than one isotope, but not that many more. Complicating the situation somewhat is the fact that there are isobaric overlaps where more than one element can possess an isotope at the same nominal mass. This increases the possible number of isotopes an element can have and reduces the number of interference-free isotopes. A number of elements, including selenium, tin, xenon, barium, and others have as many as eight or more naturally occurring isotopes. And several elements including beryllium, sodium, aluminum, cobalt, arsenic, and about a dozen other less common ones, are monoisotopic, that is, they possess only one naturally occurring isotope. Quantification in ICP-MS is based upon the signal intensity of a unique mass (isotope) for each element. After considering the isobaric overlaps, every naturally occurring element except indium has at least one "unique" isotope — that is, one that is free from direct overlap from another element, and most have more than one. So, in a perfect world, quantification in ICP-MS should be as simple and unambiguous as the spectra themselves. This is, of course, not true, and the reason is that the simple elemental spectra are complicated by spectral interferences from numerous sources, mainly from polyatomic and doubly charged ions. These spectral interferences contribute nonelemental signal to the "unique" mass/intensity measurements, resulting in bias (1). Because of this potential for bias, the results cannot always be considered unambiguous. As a result, considerable quality assurance/quality control (QA/QC) generally is applied to minimize the possibility of quantitative error. For example, in commercial environmental testing laboratories following U.S. EPA methodologies, as many as a third of all "samples" analyzed by ICP-MS are dedicated to QA/QC. These are not "paying" samples and add a significant burden in time and expense to the analysis, even when everything is working perfectly.

Steve Wilbur
What is needed is a simple, reliable test to verify that a measured isotope is free from interferences in the actual sample being measured. So far this has not existed. A simple tool, applied routinely in organic MS such as gas chromatography (GC)–MS, is the use of qualifier ions to confirm the identity or quantity of the primary or quant ion. In organic MS, in which the spectrum of a particular compound can contain many ions, the possibility of interferences on some of these ions from other coeluting compounds in the sample is significant. In this case, the "purity" of the spectrum can be checked by looking at multiple ions, which should exist in predictable ratios for pure compounds. Once the purity has been established, then quantification can be performed based upon the intensity of a single quant ion. This approach is practical because most organic MS techniques use a chromatographic step (GC or liquid chromatography [LC]) to eliminate most interfering compounds before MS analysis. Because ICP-MS in its conventional form lacks a means to eliminate interfering elements or compounds from the sample as analyzed, it is the presence of these interferences, which can overlap the quant ion, the qualifier ion, or both, that has made it difficult to use qualifier ions to confirm analyte purity and concentration.

Figure 1
Universal Helium Collision Mode and the Use of Qualifier Ions
The most common spectroscopic interferences in ICP-MS affect the first row transition elements between scandium and selenium. Sc, Co, and Mn, and As are monoisotopic, but the other elements all possess at least two isotopes that are free from isobaric overlaps. If the other spectroscopic interferences can be removed, then these second isotopes can be used as qualifier ions. However, to be effective for multielement analysis in variable or unknown samples, all interferences must be removed simultaneously, under the same conditions, and no new interferences can be formed. Only one interference removal technique in ICP-MS meets all these criteria, and that is collision cell technology using helium gas and kinetic energy discrimination (KED). Collision–reaction cell (CRC) techniques that use reactive gases such as hydrogen, ammonia, or methane do not universally remove all interferences under a single set of conditions and can cause reactive loss of analyte ions and create new interferences.
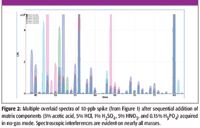
Figure 2
Two Approaches to Qualifier Ion Confirmations
If at least two isotopes for a given element can be measured without interference, then it is possible to compare the measured ratio with the expected naturally occurring isotope ratio. A good match means that interferences are not likely to be present on either isotope. Figure 1 shows a 10-ppb multielement spike into a clean matrix (0.1% nitric acid) without using a collision cell to remove interferences. The normalized expected isotope ratios are overlaid on the actual data, and the fit is excellent, indicating that in a clean matrix, without interferences, excellent agreement between primary and secondary ion abundances is possible.

Figure 3
However, when the matrix is made more complex, the isotopic agreement is poor for every element when no collision cell is used, as in Figure 2. In Figure 2, the same 10-ppb spike was fortified incrementally with the additional matrix components of 5% acetic acid, 5% HCl, 1% H2SO4, 5% HNO3, and 0.15% H3PO4. These common matrix components do not contribute to the elemental spectrum directly, but rather are the source of polyatomic interferences based upon carbon, chlorine, sulfur, nitrogen, and phosphorus. The interference contribution from each additional matrix component is overlaid on the unfortified spectrum. It is clear that there are interferences on all elements in the combined matrix.

Figure 4
A second, easier approach, which can be done automatically as part of the sample measurement, is to simply calibrate and quantify both isotopes and compare the quantitative results. Because naturally occurring isotope ratios for nearly all elements are constant and known, quantification can be performed on any isotope that is free from isobaric interference. Figure 3 shows an example of two calibration curves for copper, one based upon 63Cu and the other on 65Cu. In the absence of interferences, either can be used. Note that the spectrum for the calibration standard (Figure 3 [top]) shows good agreement with the theoretical isotopic pattern, indicating that there are no interferences on either isotope. Therefore, measuring a sample using these calibrations should yield the same result for both isotopes, if there are no interferences present. If interferences are present, the result for the interfered isotope will be higher than the noninterfered isotope. We can use this concept to construct a simple, accurate test for the presence of interferences on either (or both) ions by calculating the relative percent difference (RPD) in the quantitative result for both ions using the equation:
RPD = 100 × (Rqual – Rquant)/Rquant
where Rqual = qualifier (secondary) ion result, and
Rquant = quantifier (primary) ion result
If the RPD is zero (or near zero), then there are no interferences on either isotope and the result from either can be used. In this case, the primary ion would be used because it was selected based upon the better detection limit, most likely due to its greater relative abundance. If the RPD is positive, then an interference is present on the qualifier ion (or on both, but the effect is larger on the qualifier ion). If the RPD is negative, then the interference is on the quant ion. In any case, an RPD of close to zero would be an excellent internal check that the quantitative result has not been biased by polyatomic interferences.

Figure 5
Experimental
To test the effectiveness of using helium collision mode to permit the use of qualifier ions, several high matrix samples and reference materials were measured at both primary (quant) and secondary (qualifier) isotopes. A model 7700x ICP-MS instrument (Agilent Technologies, Santa Clara, California) was used, and the collision cell was operated in both helium mode and no-gas mode for all isotopes of all elements measured. Figure 4 shows the results obtained from 1/10 diluted seawater measured in this way (2). The error (bias) in parts per billion from the expected value is plotted on the y-axis for each of 12 elements plotted on the x-axis. Results for both the quant (isotope 1) and qualifier (isotope 2) are shown for elements in which there are two available isotopes. In helium mode, both isotopes showed less than 0.1 ppb error for all elements except Se, which showed interference at mass 82. This is due to the presence of 81BrH, which is not removed effectively by He mode. In contrast, no-gas mode (cell not used) shows significant error on one or both isotopes of Ti, V, Cr, Ni, Cu, Zn, Ga, As, and Se.
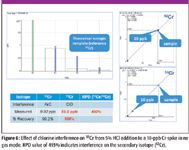
Figure 6
In a simple example of the effectiveness of using relative percent difference as a test for interferences, chromium isotopes 52 and 53 were measured in both no gas mode and helium collision mode in the presence of HCl (which interferes with 53Cr as ClO+), and methanol (which interferes with 52Cr as ArC+) and the relative percent differences calculated in all cases.

Figure 7
In Figure 5, 10 ppb Cr was spiked into a solution containing 1% methanol with the cell turned off. The ArC+ interference is apparent in the spectrum as elevated 52Cr and the RPD of –50.79% indicates a significant interference on the primary quant ion. Figure 6 shows the spectrum of 10 ppb Cr spiked into 5% HCl with the cell turned off. This time, the interference is on the secondary ion, 53Ce from ClO+, resulting in a calculated RPD of 493%. Figure 7 shows a 20-ppb spike into a matrix containing both HCl and methanol, but this time, the collision cell is pressurized with helium to remove all polyatomic interferences. In this case, the recovery is excellent for both isotopes (105% and 104%, respectively), and the RPD value is –0.84%. Either ion could be used for accurate quantification that is confirmed by the other ion.
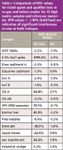
Table I
Applying the RPD test to several real-world samples and standard reference materials gave similar results. Table I shows the relative percent difference between nickel isotopes 60 and 62 for 15 different sample types including EPA interference check solutions (ICS-A, ICS-AB) in both no gas mode and helium collision mode. In no gas mode, seven samples resulted in RPD values greater than ±20%, while no samples in He mode showed RPD values greater than 4% and most were around 1%. Table II shows similar trends for Cu 65/63. Other elements show the same trends. In all cases, interferences in no-gas mode that would preclude the reliable use of qualifier ions are eliminated in helium mode, allowing qualifier ions to be routinely used to confirm quant results. Table III shows the results of the analysis of the Dutch certified reference soil, FENELAB, using He mode for 30 elements and monitoring both recovery of certified values as well as qualifier ion relative percent differences. Not only were excellent recoveries achieved, but the RPD values successfully confirmed the absence of interferences in all cases.

Table II
Unless successfully managed, polyatomic interferences can bias analytical results in ICP-MS, particularly in unknown, high matrix samples. In some cases, in relatively simple matrices, mathematical correction can correct for some polyatomic interferences, but even mathematical correction requires an interference-free correction mass to work. Alternatively, CRCs have been used successfully to remove most known interferences. However, CRCs that use reactive processes cannot remove all interferences effectively in complex matrices using a single set of conditions and therefore, cannot be used for multielement confirmation using qualifier ions. Helium collision mode, by comparison, simultaneously removes all polyatomic interferences on both quant and qualifier ions. By applying a simple test using the relative percent difference between quant results for two ions, the presence of interferences in the actual sample being measured can be determined easily. Furthermore, the mass on which the more significant interference is present is indicated by whether the RPD is positive or negative. More importantly, when the RPD value is near zero, confidence in the quantitative accuracy of the result is improved significantly. For contract laboratories, where not only the accuracy of the analysis, but also the cost is critical, this test is particularly attractive. Acquisition of the additional isotopes takes only a few seconds, because typical integration times are on the order of 100–300 ms/isotope. And because all isotopes are acquired in helium mode, time used for measuring additional isotopes is regained by eliminating the need for multiple cell modes. Furthermore, increased confidence in quantitative results can eliminate the need for re-analysis of problem samples in which the quantification was questionable.

Table III
Steve Wilbur is a senior applications scientist in the ICP-MS group at Agilent Technologies. He is part of a small, but ardent group of fanatics worldwide who believe we will ultimately build the "perfect" mass spectrometer. In the meantime, he spends his time thinking about both living with and eliminating the current "imperfections." The views expressed in this column are his and do not represent those of Agilent Technologies.
Ed McCurdy is with Agilent Technologies, Santa Clara, California.
References
(1) S. Wilbur, Spectroscopy 23(5), 18–23 (2008).
(2) Data courtesy of Wim Proper, Eurofins Analytico, Barneveld, Netherlands.

Applications of Micro X-Ray Fluorescence Spectroscopy in Food and Agricultural Products
January 25th 2025In recent years, advances in X-ray optics and detectors have enabled the commercialization of laboratory μXRF spectrometers with spot sizes of ~3 to 30 μm that are suitable for routine imaging of element localization, which was previously only available with scanning electron microscopy (SEM-EDS). This new technique opens a variety of new μXRF applications in the food and agricultural sciences, which have the potential to provide researchers with valuable data that can enhance food safety, improve product consistency, and refine our understanding of the mechanisms of elemental uptake and homeostasis in agricultural crops. This month’s column takes a more detailed look at some of those application areas.