Vibration-Resistant FT-IR for In-Process Monitoring of an Industrial Fermentation Process
Special Issues
A vibration-resistant FT-IR spectrometer is used to monitor an industrially relevant fermentation process.
A vibration-resistant FT-IR spectrometer is used to monitor an industrially relevant fermentation process. The production of two alcohols is monitored in real time along with the consumption of the sugar feedstock with concentrations ranging from 0.1 to 25 g L-1, and is fitted to HPLC data using PLS methodology.
In situ chemical monitoring of chemical reactions is one of the most powerful tools in a modern spectroscopist’s arsenal. In fact, some chemical processes can only be truly controlled if the reaction state is known in real time. However, standard FT-IR analytical instruments are highly sensitive to vibration, and often cumbersome, making it a challenge to use them in a production environment. Here we report the use of the vibration-resistant Keit FT-IR spectrometer for in-process monitoring of an industrial fermentation process. The spectrometer was used to monitor the production of the primary alcohol product, along with a secondary alcohol product and the consumption of the sugar feedstock.
Experimental Conditions
The Keit FT-IR spectrometer was incorporated into a lab-scale reactor assembly, with the reaction mixture fed through peristaltic pumps. The spectrometer was placed directly next to the pumps as their inherent vibration is not of concern for this instrument. The spectrometer was fitted with a flow cell mounted onto a dip probe featuring an AMTIR ATR crystal. Spectra were recorded every 2 h over a period of 30 h, and an aliquot of the reaction mixture was removed for HPLC testing concurrently.
Partial least square (PLS) regression method was used for a calibration and prediction model for the produced alcohols and consumed sugars. The spectral region from 900 to 1400 cm-1 was used for developing the model. The data was pre-processed with mean centering and three LVs were used to build the model.
Results and Discussion
Both the HPLC and spectral data for the reaction run is shown in Figure 1. The results clearly show that the spectroscopic data follows the HPLC data very closely, with minimal deviations between the two. Both alcohol species are observed to increase in concentration over the entire process, with a concentration range of 0.1 through to 9 g L-1. Moreover, the R2 values for correlations of predicted versus measured concentrations of alcohol 1, alcohol 2, and sugar feedstocks were 0.923, 0.912, and 0.983, respectively.
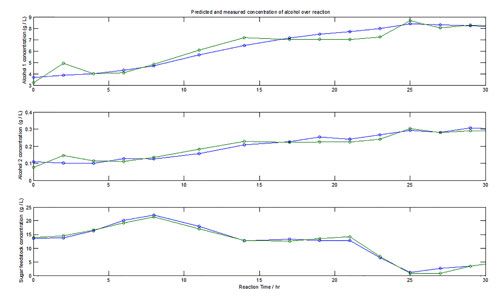
Figure 1: Reaction pathway for measured (blue) and predicted (green) concentrations of primary and secondary alcohol products, and sugar feedstock.
CLICK FIGURE TO ENLARGE
Conclusions
These results show that the Keit FT-IR spectrometer can be effectively used to monitor an industrial process in operando, with the ability to track the concentration of at least three different constituents simultaneously, and over a large range of sensitivity. The Keit FT-IR spectrometer helps improve quality, reduce waste, and improve facility utilization using a more robust and stable instrument.
Keit Spectrometers
R71, Harwell Campus, Didcot, Oxfordshire, OX11 0QX, UK
tel. +44 (0) 1235 567176
Website: www.keit.co.uk

Best of the Week: AI and IoT for Pollution Monitoring, High Speed Laser MS
April 25th 2025Top articles published this week include a preview of our upcoming content series for National Space Day, a news story about air quality monitoring, and an announcement from Metrohm about their new Midwest office.
LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.