A Comprehensive Review of Spectroscopic Techniques for Lithium-Ion Battery Analysis
Lithium-ion batteries (LIBs) are critical for a wide range of applications, including consumer electronics, electric vehicles, and renewable energy storage systems. Enhancing the performance, safety, and lifespan of LIBs requires the application of various analytical techniques across the LIBs creation and utilization stages of research and development, manufacturing, performance testing, quality control, safety testing, and recycling/remediation. Among analytical techniques used, spectroscopic methods play a pivotal role in the characterization and evaluation of LIB materials. Commonly used spectroscopic techniques in LIB analysis include inductively coupled plasma-mass spectrometry (ICP-MS), inductively coupled plasma-optical emission spectrometry (ICP-OES), micro-discharge optical emission spectroscopy (MDOES), Raman spectroscopy, X-ray fluorescence (XRF), X-ray photoelectron spectroscopy (XPS), Fourier transform-infrared spectroscopy (FT-IR), near-infrared spectroscopy (NIR), ultraviolet-visible spectroscopy (UV-vis), fluorescence spectroscopy, and nuclear magnetic resonance (NMR) spectroscopy. These analytical tools are essential for elucidating the structural, compositional, and electrochemical properties of materials used in LIBs, thereby contributing significantly to the advancement of battery performance, safety, and longevity. This review provides an overview of LIB technology, and the spectroscopic techniques employed in LIB analysis.
Key Aspects of Lithium-Ion Batteries (LIBs)
LIBs are rechargeable energy storage devices widely used in applications ranging from portable electronics to electric vehicles. The construction of LIBs involves several key components and a systematic manufacturing process. To begin this discussion, we look at an outline including components, construction steps, and the analytical methods used across the research, development, manufacturing, quality control, safety testing, and recycling stages.
LIB Battery Components
Included here is a list of the main components of LIB structures and essential details about each component (1–5). For an illustration of a LIB see Figure 1.
FIGURE 1: Principles of lithium-ion battery (LIB) operation: (a) schematic of LIB construction showing the various components, including the battery cell casing, anode electrodes, cathode electrodes, separator (insulator) layers, electrolyte solution, and positive and negative battery terminals; (b) During discharge, lithium ions (Li+) move from the anode electrode to the cathode electrode through the electrolyte, while electrons (e−) travel through the external circuit from the anode electrode to the cathode electrode, powering a device. During charging, this process reverses for both Li+ and e−. The anode electrode (negative terminal during discharge) stores lithium and undergoes oxidation, while the cathode electrode (positive terminal during discharge) undergoes reduction. The external terminals connect the battery to a device and are attached to the bank of corresponding electrodes where the electrochemical reactions occur. The electrolyte solution in a lithium-ion battery typically contains lithium hexafluorophosphate (LiPF6) dissolved in a mixture of organic carbonates, enabling efficient lithium ion movement between electrodes while ensuring stability and high ionic conductivity for optimal battery performance and safety (Figure courtesy of the author).
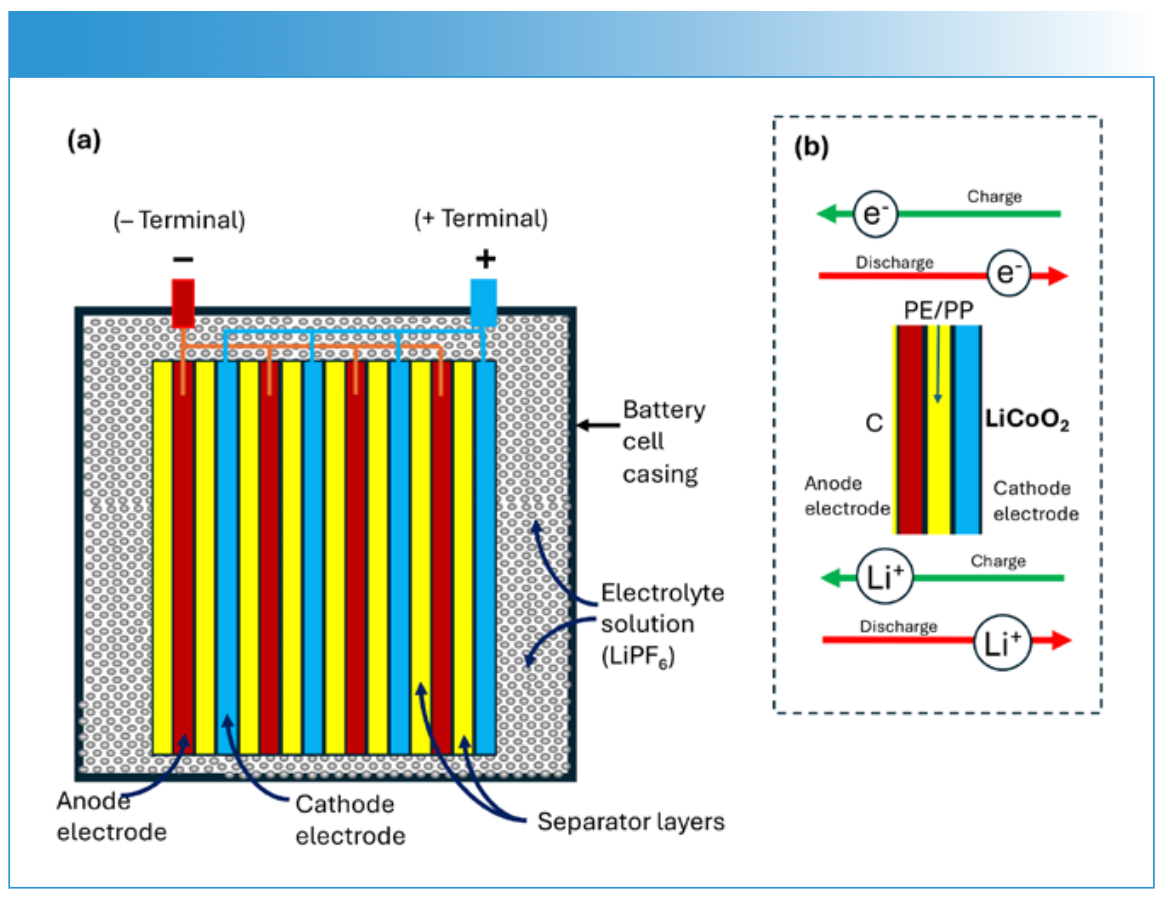
- Cathode: The positive electrode, usually made from lithium metal oxides, such as lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), lithium nickel manganese cobalt oxide (NMC), and lithium nickel cobalt aluminum oxide (NCA).
- Anode: The negative electrode, typically composed of graphite (carbon-based materials), though silicon and lithium titanium oxide (LTO) are also under investigation for improvements.
- Electrolyte: A liquid or gel medium that facilitates ion transfer between the electrodes. Common electrolytes are lithium salts like LiPF6 dissolved in organic solvents (ethylene carbonate and dimethyl carbonate).
- Separator: A porous polymer membrane (polyethylene or polypropylene) that prevents physical contact between the cathode and anode while allowing the passage of ions.
- Current Collectors: Conductive materials (copper for the anode and aluminum for the cathode) that collect electrons for external circuit flow.
- Battery Cell Casing: The outer structure that protects the battery and houses the components. The casing is typically made from aluminum, steel, plastic (commonly used in pouch cells), or composite materials, such as, for example, carbon fiber reinforced polymers (CFRP), glass fiber reinforced polymers (GFRP), polymer blends, or metal matrix composites (MMC).
LIB Construction Steps (1–5)
- Electrode Preparation: Is composed of a cathode slurry, which is a mixture of active material (LiCoO2), a conductive agent (carbon black), and a binder (polyvinylidene fluoride, PVDF) is made into a slurry and coated onto an aluminum foil. The anode slurry is made with a similar process as for the anode, where graphite, conductive agents, and binders are coated onto copper foil.
- Drying and Calendering: The coated foils are dried to remove solvents, followed by calendering (rolling) to adjust the thickness and density of the electrode layers.
- Electrode Cutting and Stacking: The electrodes are cut into the desired shape and size. They are either stacked or wound into a cylindrical or prismatic form, depending on the battery design.
- Cell Assembly: The electrodes are assembled with a separator in between them. This is done in a dry, inert atmosphere to prevent contamination and moisture uptake.
- Electrolyte Filling: The electrolyte is injected into the cell, ensuring that it thoroughly wets the separator and electrodes.
- Sealing: The cell is sealed using heat or crimping to prevent electrolyte leakage and contamination by external air or moisture.
- Formation Process: The battery undergoes several charge/discharge cycles to stabilize its internal chemistry. This step helps to form the solid electrolyte interphase (SEI) layer on the anode, which is crucial for long-term stability and performance.
Important Electrical Analysis Parameters for LIBs (1–10)
The key analysis parameters measured for battery health and functionality are given in the following list. These are not directly measured using classical spectroscopy techniques, but are included here for reference purposes.
- Capacity: Measured in ampere-hours (Ah), it reflects the battery’s energy storage.
- Energy Density: Indicates how much energy can be stored per unit volume or weight (Wh/kg or Wh/L).
- Power Density: Represents how much power (W/kg) the battery can deliver.
- Cycle Life: The number of charge/discharge cycles a battery can undergo before its capacity falls below 80% of the original.
- Coulombic Efficiency: The ratio of charge output to charge input in a cycle, ideally close to 100%.
- Voltage Window: The operating voltage range for optimal performance and safety.
- Internal Resistance: Impacts efficiency and heat generation during charging or discharging.
- Thermal Stability: The temperature at which the battery components remain safe without degradation.
- State of Health (SoH): Reflects the overall health and performance of a battery after usage.
Analytical Methods Used for LIBs Analysis Applications (20–30)
A variety of spectroscopic techniques are used for analysis of the various battery components and for the different stages of battery life. Here is a categorized breakdown for each analytical method applied to lithium-ion battery (LIB) analysis across different stages such as research and development (R&D), manufacturing, performance testing, quality assessment, and remediation and recycling:
Research and Development (R&D)
The analytical techniques and applications for the development of new materials and battery chemistries are as follows.
- ICP-MS/ICP-OES: Measures trace metal impurities in novel electrode and electrolyte materials to ensure high performance and minimize degradation.
- Raman Spectroscopy: Investigates molecular structure and bonding in newly developed cathode/anode materials, monitoring phase changes during cycling.
- XRF: Determines the elemental composition of new cathode materials to optimize battery performance and lifespan.
- XPS: Analyzes the surface chemistry of new materials, identifying oxidation states and chemical environments for electrode materials.
- FT-IR and NIR: Analyzes the molecular structure of electrolyte additives to enhance ionic conductivity and stability.
- UV-vis Spectroscopy: Examines the optical properties of new electrolyte additives, including light absorption to predict photodegradation.
- Fluorescence Spectroscopy: Detects low levels of fluorescent degradation byproducts in new electrolyte formulations.
- NMR Spectroscopy: Studies the local chemical environments and ion mobility in novel electrolyte systems to improve conductivity and thermal stability.
Manufacturing
The analytical techniques and applications for LIB production and quality control are as follows.
- ICP-MS/ICP-OES: Ensures the purity of raw materials used in electrode manufacturing by detecting metal contaminants.
- μDOES: Monitors electrode composition during the manufacturing process to ensure consistent material properties.
- Raman Spectroscopy: Verifies the crystalline structure of electrode materials during production, ensuring no phase transitions have occurred.
- XRF: Provides fast, non-destructive testing of electrode materials to confirm proper elemental composition.
- FT-IR: Monitors the composition of binders and electrolytes during the manufacturing process.
- UV-vis Spectroscopy: Measures the stability of electrolyte additives during the production process.
Performance Testing
The analytical techniques and applications for assessing the operational characteristics of LIBs are as follows.
- ICP-MS/ICP-OES: Assesses the leaching of metals from electrodes into the electrolyte during battery cycling, which can affect battery life.
- Raman Spectroscopy: Monitors phase transitions in electrode materials during cycling, correlating with capacity fade and performance issues.
- XPS: Evaluates changes in surface chemistry on electrodes during cycling, particularly solid-electrolyte interphase (SEI) formation.
- FT-IR: Tracks the formation of degradation products in the electrolyte during battery cycling.
- NMR Spectroscopy: Analyzes the mobility of lithium ions within the electrolyte during battery cycling to evaluate ion diffusion performance.
Quality Assessment
The analytical techniques and applications for ensuring the quality and consistency of LIB materials are as follows.
- ICP-MS/ICP-OES: Performs routine quality checks for trace metal impurities in electrode and electrolyte materials.
- Raman Spectroscopy: Checks for consistent crystallinity and phase purity in the cathode/anode materials across production batches.
- XRF: Ensures that the elemental composition of materials is within specification, preventing variability in battery performance.
- XPS: Inspects surface contaminants on electrodes that can negatively affect battery efficiency and longevity.
- FT-IR and NIR: Assesses the purity of electrolytes and binders to ensure no organic impurities are present.
- UV-vis Spectroscopy: Confirms the uniformity of light-sensitive electrolyte components to prevent photodegradation.
Remediation and Recycling
The analytical techniques and applications for disposal of spent LIB materials are as follows.
- ICP-MS/ICP-OES: Used to analyze recovered materials (metals like lithium, cobalt, and nickel) from spent batteries to evaluate their purity and suitability for reuse.
- XRF: Monitors the composition of recycled cathode materials.
- Raman Spectroscopy: Characterizes the structure of reclaimed electrode materials, ensuring they retain their crystalline properties after recycling.
- XPS: Evaluates the surface composition of recycled electrodes, checking for contamination or unwanted phases.
- FT-IR and NIR: Detect residual organic contaminants from electrolyte materials in the recycling process.
- NMR Spectroscopy: Assesses changes in the chemical structure and degradation of electrolyte components during recycling.
Detailed Summary of Spectroscopic Techniques Used for LIBs Analysis
The following summaries of each analytical technique include an overview of the technique, its main applications for LIB analysis, and recent research papers highlighting the use of the technique for LIB analytical assessment.
Inductively Coupled Plasma-Mass Spectrometry (ICP-MS)
ICP-MS is a highly sensitive technique used for the elemental analysis of materials. It measures the mass-to-charge ratio (m/z) of ions generated by an inductively coupled plasma, providing precise quantification of trace elements in final components and raw materials used for manufacturing components.
Applications
- Trace Element Analysis: ICP-MS is crucial for detecting trace impurities cathode materials, and electrolyte components. Elemental analysis is performed in battery materials, such as concentrations of lithium, cobalt, and nickel. Ensuring low levels of contaminants is vital for maintaining battery performance and safety.
- Isotope Ratios: This technique can analyze lithium isotopes, which are important for understanding the electrochemical behavior and reactions within the battery.
ICP-MS Research
Lithium-ion batteries, introduced 25 years ago, have quickly become the dominant power source for portable electronics, and a leading candidate for large-scale energy storage applications, such as electric vehicles and grid electricity storage. A primary challenge with these batteries is degradation over time, known as aging, which reduces both calendar life (storage capacity over time) and cycle life (capacity over repeated use). The aging process arises from numerous effects on both individual cell components and their interactions, necessitating a variety of analytical techniques to investigate these issues. Elemental analysis plays a crucial role in studying the battery constituents and their degradation products, providing essential data to mitigate aging effects. By examining the elemental composition and its changes using such primary techniques as ICP-MS and ICP-OES, researchers aim to improve the performance and longevity of lithium-ion batteries, advancing their viability in applications like electric mobility, stationary storage, and grid energy systems. This review highlights the use of elemental analysis techniques in understanding the degradation mechanisms of lithium-ion battery materials (11).
Lithium-nickel-manganese-cobalt oxides (NCMs) are widely used as cathode materials in lithium-ion batteries. Large-scale production of NCM precursors is typically performed through coprecipitation in continuous stirred-tank reactors (CSTR). However, this process often results in broad particle size distributions and compositional heterogeneity both within and between particles. To quantify these variations, advanced analytical methods are needed. In one study, single particle laser ablation-inductively coupled plasma-mass spectrometry (spLA-ICP-MS) was employed to investigate particle size-dependent elemental compositions in CSTR-produced NCM precursors (12). The results showed a significant enrichment of nickel in larger particles, while cobalt and manganese were enriched in smaller particles. These compositional differences were maintained even after calcination with lithium hydroxide. The particle size-dependent concentration gradients detected by spLA-ICP-MS were further validated using scanning electron microscopy (SEM) coupled with energy-dispersive X-ray analysis (SEM-EDX), confirming the reliability of the technique (12).
Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
ICP-OES is used for the elemental analysis of materials by measuring the light emitted by atoms in a plasma state. This technique provides qualitative and quantitative data on the elemental composition of raw materials and assembled components.
Applications
- Elemental Composition: ICP-OES is often used to analyze the composition of electrode materials and electrolytes, ensuring they meet manufacturing specifications. Similar to ICP-MS but better suited for higher concentration ranges. Used to analyze metallic elements in electrode materials, ensuring proper composition.
- Contaminant Detection: Similar to ICP-MS, ICP-OES can identify trace metals and impurities that may affect battery performance.
ICP-OES Research
Lithium carbonate (Li2CO3) is essential for cathode material production in lithium-ion batteries, where impurity levels can significantly impact battery performance and longevity. However, detailed studies on the trace elemental analysis of Li2CO3 salts are limited. One study established and validated a comprehensive analytical methodology to detect and quantify impurities in Li2CO3 using a combination of techniques (13).
X-ray diffraction (XRD) was employed to determine the phase composition of Li2CO3, revealing the presence of the zabuyelite phase, the natural form of lithium carbonate. Scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDX) provided insights into particle morphology and revealed trace impurities of Si and Al, quantified at 0.12% and 0.14%, respectively. X-ray photoelectron spectroscopy (XPS) further identified surface impurities, including S, Cr, Fe, Cl, F, Zn, Mg, and Na, alongside Li, O, and C (13).
ICP-OES was used to quantify trace elements after acid digestion and dilution of lithium salt samples. This method demonstrated excellent linearity (R² > 0.99) and sensitivity, with limits of detection (LOD) ranging from 0.001 to 0.800 ppm and limits of quantification (LOQ) from 0.003 to 1.1 ppm, depending on the element. The recovery rate for the analyzed elements exceeded 90%, confirming the precision of the method (13).
The combination of XRD, SEM-EDX, XPS, and ICP-OES provided a robust approach for identifying and characterizing impurities in Li2CO3, with the ICP-OES method proving especially useful for trace-level detection and quantification (13).
Micro-Discharge Optical Emission Spectroscopy (μDOES)
μDOES is a miniaturized optical emission spectroscopy technique that generates a localized plasma discharge to analyze the elemental composition of materials. It provides rapid, non-destructive analysis and is particularly effective for in-line monitoring and quality control during manufacturing.
Applications
- In-line Process Monitoring: μDOES is used to monitor electrode material composition during the manufacturing process, providing real-time data to ensure material consistency and quality.
- Elemental Composition: It verifies the elemental makeup of electrode and cathode materials, ensuring they meet the required specifications for battery performance.
- Surface Contaminant Detection: Detects surface contaminants on electrode materials, which can degrade battery performance or lead to premature failure.
- Quality Control: μDOES is implemented in manufacturing lines to continuously check for impurities or compositional variations in raw materials or finished electrodes, ensuring product uniformity.
μDOES Research
The increasing demand for high-purity battery materials and the need for precise detection of trace metal impurities have driven the development of new analytical methods for both battery production and recycling. Traditional techniques like laboratory ICP-OES and ICP-MS, though accurate, are limited to stationary laboratory use due to their high resource demands, making them unsuitable for real-time, on-site industrial process monitoring. One study investigates the application of micro-discharge optical emission spectroscopy (μDOES) for the online quantification of trace metal impurities in lithium salt solutions. μDOES employs a micro-plasma generated directly in the aqueous sample without carrier gas, using electrodes and high voltage pulses, allowing for fast and precise analysis (14).
In one study, impurity elements (Na, K, Al, Fe, and Zn) were measured simultaneously in lithium carbonate and lithium hydroxide solutions with lithium concentrations ranging from 0.3 to 2 mg/L and impurity concentrations from 0 to 50 μg/L (14). Calibration and long-term stability measurements were conducted, optimizing parameters such as plasma discharge energy and sample conductivity. The technique demonstrated a relative standard deviation of 3% for lithium concentration stability over 9 h, with detection limits in the low μg/L range, comparable to ICP-OES. Multilinear regression models were applied to correct for ionization effects at higher lithium concentrations. On-site industrial samples were analyzed, and the results were validated against laboratory ICP-OES, confirming μDOES as a reliable method for real-time trace metal analysis in lithium matrices (14).
Raman Spectroscopy
Raman spectroscopy involves the inelastic scattering of monochromatic light (usually from a laser) off molecular vibrations. This technique is sensitive to molecular symmetry and can provide information about vibrational modes in materials.
Applications
- Structural Properties of Materials: This includes analysis of SEI layer formation, phase transitions in electrodes, and degradation mechanisms. It’s also useful in recycling for characterizing materials in spent batteries.
- Phase Identification: Raman spectroscopy is essential for identifying different phases in electrode materials, such as graphite and silicon-based anodes. It can differentiate between crystalline and amorphous structures.
- Electrochemical Behavior: In situ Raman spectroscopy can monitor changes in material properties during charge and discharge cycles, revealing insights into degradation mechanisms and structural changes.
Raman Research
Understanding inhomogeneous charging and discharging reactions in electrodes is crucial for enhancing the rate capability of lithium-ion batteries. In one study, a simplified method was developed to observe reaction distribution within graphite composite electrodes using operando Raman spectroscopy. Specially designed cells enabled real-time comparison of electrode behavior on both the electrolyte and current collector sides. Electrodes with varying densities and thicknesses were analyzed (15).
For low-density electrodes, changes in graphite structure occurred nearly simultaneously on both sides, with little dependence on electrode thickness. However, as electrode density increased, the reaction distribution became dependent on thickness, suggesting that ion transport length plays a key role when ion channels between the current collector and electrolyte sides are reduced by higher electrode density (15).
The operando Raman spectroscopy technique provides a straightforward approach for investigating reaction distribution within electrodes, offering valuable insights into the impact of electrode density and thickness on lithium-ion transport (15).
The solid-electrolyte interphase (SEI) is critical for lithium metal batteries due to its influence on lithium deposition and dissolution, which directly affects battery performance. A depth-sensitive plasmon-enhanced Raman spectroscopy (DS-PERS) method was developed to enable in situ, nondestructive characterization of the SEI’s nanostructure and chemical composition using synergistic plasmonic structures. However, the mechanisms behind the signal enhancement remained unclear (16).
Through a combination of theoretical modeling and experimental techniques, this research reveals that “hot spots” generated by the plasmonic structures are essential for detecting Raman signals at different stages of SEI developments. Initially, these hot spots form at the copper (Cu) interface but shift to the lithium (Li) interface as Li deposition progresses. As the deposited Li thickens, the localized surface plasmon resonance from Cu becomes shielded, causing the hot spots from Li-Li coupling to diminish. To address this, Cu@Li multilayer shell-isolated nanoparticles were introduced, creating new hot spots that enhance depth sensitivity.
The results provide essential theoretical insights into optimizing depth-resolved DS-PERS for precise SEI characterization, significantly advancing the understanding of lithium metal battery behavior (16).
X-ray Fluorescence (XRF)
XRF is an analytical technique used for elemental analysis by measuring the characteristic X-rays emitted from a sample when it is excited by a primary X-ray source.
Applications
- Non-Destructive Elemental Analysis of Electrode Materials: It is used in quality control to confirm the composition of cathode and anode materials.
- Elemental Composition: XRF can quickly analyze the elemental composition of electrodes, electrolytes, and other battery components, providing valuable data for quality control.
- Coating Thickness: This technique is useful for monitoring the thickness of electrode coatings, ensuring uniformity and consistency in manufacturing.
XRF Research
To ensure the stability of cathodes at high voltages (greater than 4.3 V vs. Li/Li⁺), it is essential to implement particle-scale surface protection. However, there has been limited systematic study on the effect of nanoscale coating coverage on cathode particle surfaces and their stability. Recent research presents a quantitative analysis of how coating homogeneity affects the stability of cathode particles under high voltage conditions (17).
A metal alkoxide precursor-based coating methodology was employed, leveraging the pH dependence of the zeta potential to manipulate the coating structure and adjusting the precursor evaporation rate. Ta-substituted Li7La3Zr2O12 was selected as the coating material for a lithium transition metal oxide, such as Li(Ni1/3,Co1/3,Mn1/3)O2 cathode particles, with the coating structure varied while maintaining consistent coating concentration. The coating structures were characterized using mainly X-ray fluorescence (XRF), with X-ray photoelectron spectroscopy (XPS), and electrochemical impedance spectroscopy (EIS) (17).
The results indicated that cathode particles with more homogeneous coatings demonstrated significantly improved cycle stability and reduced charge transfer resistance at potentials exceeding 3.9 V. This study highlights that optimizing coating homogeneity can substantially enhance battery performance, providing valuable insights for the development of more efficient lithium-ion batteries (17).
The management of end-of-life (EoL) batteries, particularly lithium-ion batteries (LIBs), has become a critical focus in the pursuit of sustainable energy solutions and environmental protection. As reliance on batteries increases, there is a pressing need for efficient recycling methods that recover valuable materials while minimizing environmental impact and promoting a circular economy (18).
A literature review assessing the LIB market, estimated return volumes, and current sorting and recycling processes has been published (18). Additionally, a manual dismantling and input analysis of consumer LIBs were performed. The findings indicate that existing recycling processes are limited to individual cathode active materials, lacking effective sorting mechanisms for pre-sorted LIBs (18).
The study proposes that techniques such as X-ray transmission (XRT), XRF, and optical sorting could potentially differentiate LIBs based on their cathode active materials. However, to substantiate this hypothesis, further investigations are necessary. These advancements are essential for developing an effective sorting process that can enhance recycling efficiency and contribute to sustainable battery management practices (18).
X-ray Photoelectron Spectroscopy (XPS)
XPS is a powerful analytical tool used extensively in the research, development, manufacturing, performance testing, and quality assessment of lithium-ion batteries (LIBs). This technique provides detailed chemical information about the surface composition, oxidation states, and electronic environment of battery materials, particularly crucial for understanding electrode behavior.
Applications
- Surface Composition Analysis: XPS can be used to determine the elemental composition and chemical state of the materials on the surface of LIB electrodes, such as lithium, oxygen, carbon, and transition metals (for example, nickel, cobalt, and manganese).
- Interfacial Layer Characterization: XPS is crucial for analyzing the solid electrolyte interphase (SEI) layer, which forms on the anode surface during the battery’s initial cycles. It helps to identify the chemical compounds present in the SEI.
- Oxidation State Determination: XPS can provide information on the oxidation states of the transition metal components in the cathode materials. This is essential for understanding the electrochemical performance and stability of the cathodes.
- Degradation and Failure Analysis: XPS can be employed to study surface degradation mechanisms and the presence of unwanted species (such as electrolyte decomposition products) that form during cycling, helping in failure analysis of LIBs.
XPS Research
The development of silicon oxycarbide (SiOC) anodes for LIBs has been hindered by challenges such as limited reversible capacity, inadequate rate performance, and complex fabrication methods. A novel one-pot synthesis strategy that enables the in situ construction of SiOC with oxygen-rich structural units and a flake morphology, thereby improving the reversible capacity and lithium ion transport capability, has been developed (9).
The synthesized SiOC electrodes demonstrate a reversible capacity of 906 mAh/g at a current density of 0.1 A/g, along with a notable rate capability of 591 mAh/g and a capacity retention rate of 98% at 2.0 A/g after 1200 cycles. Characterization of the SiOC structure reveals that a higher content of oxygen-rich units and flake morphology promotes the formation of a stable solid-electrolyte interphase (SEI) and mitigates volume expansion during cycling. Additionally, the presence of LixSiOy compounds in the SiOC anodes contributes to reduced irreversible lithium ion consumption during initial charge cycles (19).
The material characterization techniques employed in this research strategy include (19):
- X-ray photoelectron spectroscopy (XPS) for surface chemical analysis.
- 29Si solid-state nuclear magnetic resonance (29Si SS-NMR) to investigate silicon bonding characteristics.
- X-ray diffraction (XRD) to confirm the crystal phase.
- Fourier transform infrared spectroscopy (FT-IR) for functional group analysis.
- Brunauer-Emmett-Teller (BET) surface area measurements.
- Thermogravimetric analysis (TGA) and Raman spectroscopy for carbon content determination.
- Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) with energy dispersive spectroscopy (EDS) for morphological and particle size characterization.
Overall, the findings indicate that the structural manipulation and morphological enhancement of SiOC can significantly improve electrochemical performance, providing a pathway for more efficient battery anodes (19).
With the increasing severity of global energy shortages, LIBs have emerged as vital alternatives to fossil fuels. However, thermal runaway presents a significant challenge to the development of battery technology. One investigation studied the effects of natural aging and state of charge (SOC) on the thermal runaway behavior of batteries using a combustion test chamber. The thermal runaway process was characterized in four distinct stages: battery bulge, smoke release, jet fire, and fire extinction (20).
The results indicate that LiFePO4 batteries with higher SOC are more susceptible to thermal runaway, while natural aging significantly affects the intensity of the event. Gas products emitted during the fire occurrence were identified as C1–C4 hydrocarbons. Furthermore, soot particles generated during battery combustion exhibited a characteristic “core-shell” structure. Surface analysis revealed the presence of C–C, C–O, and O–H bonds in the soot, while soot from early-manufactured batteries also contained carboxylate group (O–C=O) and π bonds. In addition to carbon and oxygen, trace elements including lithium, fluorine, phosphorus, and iron were detected in the soot (20).
To characterize the soot particles, two key analytical techniques were employed (10): Energy dispersive spectroscopy (EDS) was used to determine the elemental composition of the soot, and X-ray photoelectron spectroscopy (XPS), utilizing a PHI QUANTERA-II SXM model, provided insights into elemental and functional group distributions on the soot surface, as well as the degree of carbon atom hybridization (1). XPS analysis focused on the C1s peak, enabling the identification of hydroxyl (C–OH) and carbonyl (C=O) functional groups (10). Overall, this study enhances the understanding of soot emissions during battery thermal runaway, offering critical insights for evaluating the toxicity and environmental impact of LIBs (20). Overall, XPS plays an indispensable role in advancing the development of high-performance and durable lithium-ion batteries by providing precise chemical analysis and surface characterization (20).
Fourier Transform-Infrared (FT-IR) Spectroscopy
FT-IR spectroscopy analyzes molecular vibrations within battery materials by measuring the absorption of infrared radiation. This technique provides characteristic spectral features related to molecular structure.
Applications
- Compound Identification: FT-IR is used for identification of organic and polymeric compounds in the electrolyte and separator. It can also monitor degradation of organic components during cycling and safety testing.
- Material Characterization: FT-IR is used to identify functional groups in electrode and electrolyte materials, providing insights into their chemical composition and stability.
- Interfacial Studies: It examines the formation of the solid electrolyte interphase (SEI) on electrode surfaces, which is critical for understanding battery performance and longevity.
FT-IR Research
The development of LIB technology relies heavily on a comprehensive understanding of Li-ion solvation and charge transport dynamics. One study utilizes ultrafast two-dimensional infrared (2D-IR) spectroscopy to investigate these dynamics in LIB electrolytes, specifically focusing on the chemical exchange processes through time-dependent cross-peak analysis (21).
The interpretation of 2D-IR spectra is often complicated by various factors, including vibrational energy transfer and the molecular photothermal effect (MPTE), which significantly impact the development of cross-peaks. Identifying the exact origin of these cross-peaks has proven challenging in time-resolved IR spectroscopic studies of LIB electrolytes (21).
In one research paper, the authors examine the 2D-IR cross-peaks in LIB electrolytes with acetonitrile as the solvent, analyzing mixtures of LiSCN and CH3SCN in CD3CN. The time-dependent analysis reveals distinct features related to MPTE, indicating its influence on the spectral data (21).
Furthermore, the direct observation of intermolecular MPTE is achieved through two-color IR pump–probe spectroscopy, which corroborates the findings. The results highlight the significant artifacts induced by MPTE in ultrafast dynamics studies and underscore the importance of accounting for these effects to accurately observe the charge transport processes within LIB electrolytes (21).
An investigation of methyl p-toluene sulfonate (MPTS) as a novel film-forming additive in lithium/graphite cells was published (22). Density functional theory (DFT) calculations revealed that MPTS has a more negative electron affinity energy of −2.17 eV compared to ethylene carbonate (EC) at −1.03 eV and dimethyl carbonate (DMC) at −0.73 eV, indicating its superior reductive activity. This finding aligns with cyclic voltammetry (CV) test results, supporting MPTS’s effectiveness as an additive (22).
The physicochemical characteristics of the graphite electrodes were analyzed using FT-IR spectroscopy, field-emission scanning electron microscopy (FESEM), X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM). Electrochemical testing demonstrated that the inclusion of 1.5% wt% MPTS resulted in low impedance at the electrode interface and exceptional cyclability, with the battery retaining 93.17% of its initial capacity at 0.2 C (C-rate) after 100 cycles—approximately 11% higher than batteries with conventional electrolytes (22). A battery C-rate is the rate at which a battery is charged or discharged relative to its maximum charge capacity, where 1C means charging or discharging the full battery capacity in one hour.
The enhanced battery performances are attributed to the preferential adsorption of MPTS and the formation of a sulfur-containing solid-electrolyte interphase (SEI) layer on the graphite electrode. TEM and XPS analyses confirm that this SEI layer is thinner and enriched with sulfur, which facilitates faster lithium intercalation and deintercalation kinetics by reducing charge transfer resistance. The results highlight the potential of MPTS in improving the performance of lithium-ion batteries through the optimization of the electrode interface (22).
Near-Infrared (NIR) Spectroscopy
NIR spectroscopy measures overtones and combination bands associated with molecular vibrations in the near-infrared region.
Applications
- In Situ Analysis: NIR is used for rapid, in situ non-destructive analysis of polymer separators and electrolyte components. It can measure physical and chemical changes in these materials.
- Non-destructive Analysis: NIR can rapidly analyze bulk materials, such as electrolyte solutions, without extensive sample preparation, making it efficient for quality control.
- Quality Control: It is used for online monitoring of moisture content in battery materials during production, which is essential for maintaining optimal performance.
NIR Research
An investigation of the optical characterization of graphite anodes in LIBs to develop methods for estimating their state of charge (SOC) was completed using NIR (23). The approach employs NIR reflectance spectroscopy on the anode of commercial LIB cells, alongside in situ optical measurements utilizing an embedded optical fiber sensor. The analysis measured wavelengths from 500 to 900 nm, revealing that the reflectance properties of the anode are primarily influenced by graphite rather than the solid electrolyte interface (23).
Notably, the study demonstrates that the reflectance of lithiated graphite exhibits significant variation in the near-infrared range (750–900 nm), particularly as a function of SOC, contrasting with the visible spectrum (350-750 nm). The embedded optical sensor further facilitates the measurement of transmittance within the near-infrared spectral band. These findings indicate the potential to develop an economical and unique method and instrument for accurately estimating the SOC of LIBs, leveraging the distinct optical properties of graphite anodes as they undergo lithiation (23).
An exploration into the use of NIR transmission optical microscopy, with measurement wavelengths greater than 730 nm, investigated intra-particle electrochemistry in micron-sized LiCoO2 particles. Such investigations have been previously underexamined due to methodological limitations. The strong light penetration of NIR microscopy enables the measurement of redox electrochemistry in single microparticles by analyzing optical contrast changes during electrochemical cycling (24).
Key findings of this work reveal that the solid-state diffusion within these microparticles exhibits distinct directional characteristics, deviating from the isotropic diffusion patterns typically observed in smaller particles. This directional diffusion is corroborated by dark field scattering microscopy using NIR, which suggests that non-uniform inner crystal structures contribute to the geometrically asymmetric charge transfer kinetics observed within individual particles.
Overall, the results of this research highlight the potential of NIR optical techniques for operando studies of practical battery materials, paving the way for deeper insights into the electrochemical behavior of micron-sized particles in battery applications (24).
Ultraviolet-Visible Spectroscopy (UV-vis)
UV-vis spectroscopy measures the absorption of ultraviolet and visible light by electronic transitions in molecules, providing insights into the optical properties of materials.
Applications
- Electrolyte Characterization: UV-vis spectroscopy analyzes the optical properties of electrolyte components, providing information on their stability and reactivity under operating conditions.
- Degradation Studies: This technique can monitor the degradation of organic solvents and additives in electrolytes, helping to assess their long-term stability.
UV-vis Research
This dissolution of transition-metal (TM) cations, specifically focusing on manganese (Mn) ion release from LiMn2O4 (LMO) cathodes, which negatively impacts the cycling performance of LIBs, has been published (25). Employing a refined in situ ultraviolet-visible (UV-vis) spectroscopy technique, the research monitors the concentration of dissolved Mn ions in the liquid electrolyte across various states of charge (SOC). The findings indicate that the maximum dissolution concentration and rate occur at the 4.3 V charged state, with Mn2+ identified as the predominant species in the electrolyte (25).
To complement the experimental observations, ab initio molecular dynamics (AIMD) simulations are utilized to elucidate the relationship between Mn dissolution and factors such as surface structure evolution, solvent decomposition, and lithium salt presence. This work enhances the understanding of transition metal dissolution mechanisms under operational conditions and provides insights for the design of more stable cathode materials in LIBs (25).
The escalating disposal of LIBs poses significant environmental challenges but also offers substantial opportunities for resource recovery. In a recent paper, a recycling strategy was introduced using a green, cost-effective ternary deep eutectic solvent (DES) composed of guanidine hydrochloride, ethylene glycol, and maleic acid. The DES can achieve >99.5% leach efficiency of lithium (Li) and cobalt (Co) at 100 °C, 9h, and L/S=50 (26). The kinetic study found that the chemical reaction rate at the interface was the key to controlling the leaching process. The study of leaching mechanisms found that the acidity and coordination ability of DES were the main driving forces of leaching. The leaching mechanism was extensively examined using UV–vis and FT-IR spectroscopic methods. Co loaded in the DES phase can almost precipitate out by using oxalic acid, achieving the separation of Li and Co. After reusing three times, the maximum load of Li in the DES phase reached 4.1 mg/g. These outstanding performances, along with the avoidance of the use of hazardous chemicals and the reduction of high costs, confirmed that the DES possesses a promising prospect for sustainable, large-scale application in efficiently leaching valuable metals from LiCoO2 (26).
Fluorescence (FL) Spectroscopy
Fluorescence (FL) spectroscopy detects light emitted by a material after absorbing electromagnetic radiation, providing highly sensitive, non-destructive analysis of LIB components. It is particularly effective in studying organic materials and transition metal degradation products, offering quantitative insights into complex chemical behaviors.
Applications
- Electrolyte Degradation: Monitors degradation of organic solvents and formation of byproducts.
- Cathode/Anode Material Analysis: Detects metal ion dissolution and SEI layer formation.
- Additive Identification: Tracks fluorescent additives’ distribution and performance.
- Aging & Decomposition Detection: Identifies degradation products from cycling-induced chemical changes.
- Surface Analysis: Fluorescence microscopy studies spatial degradation on electrodes.
- Redox Reaction Monitoring: Tracks fluorescence changes in redox-active materials.
FL Research
The development of a rapid, selective fluorescence detection method for lithium hexafluorophosphate (LiPF6) using metal-organic cages (MOC), with a nearly 20-fold enhancement in emission has been published (27). The technique, which offers stability and resistance to photobleaching, allows both quantitative and qualitative detection of LiPF6 and moisture in battery electrolytes. The fluorescence enhancement is attributed to a hydrogen bond-induced emission effect within the MOC (27).
Key analytical methods include fluorescent excitation-emission matrix spectra and variable-temperature NMR spectroscopy, which clarified the role of hydrogen bonding in enhancing the emission. Density functional theory (DFT) simulations were employed to model host-guest interactions and estimate adsorption energy (27). Fluorescence spectroscopy analysis showed that adding LiPF6 to the MOC in dimethylformamide (DMF) resulted in a significant increase in fluorescence emission, indicating the system’s sensitivity to LiPF6. Intramolecular hydrogen bonds in the 2-amino-terephthalic acid (2-NH2-TPA) monomer contributed to the emission enhancement, confirmed by comparing the fluorescent properties of MOC, MOC+LiPF6, and other monomer compounds. This method offers potential for designing novel host structures for specific recognition of other target compounds (27).
Lithium-sulfur (Li-S) batteries are promising for next-generation energy storage due to their high theoretical gravimetric specific capacity (1672 mAh/g) and energy density (2567 Wh/kg). However, their commercialization is hindered by the polysulfide shuttle effect, where soluble lithium polysulfides (Li2Sx, 6 ≤ x ≤ 8) diffuse from the cathode to the anode, causing parasitic reactions, rapid loss of active material, and cell degradation (28). One study utilizes operando optical fluorescence microscopy to visualize the real-time distribution and dynamics of polysulfides within optically accessible Li-S cells (28). By using a selective fluorescent dye, polysulfide concentrations were quantitatively tracked throughout the electrolyte and cathode, enabling direct observation of polysulfide shuttling to the anode and subsequent dendrite formation. This methodology provides valuable insight into the behavior of polysulfides during cycling and offers a platform to evaluate mitigation strategies, such as the use of LiNO3 additives. The technique enables real-time monitoring of polysulfide distribution, facilitating optimization of approaches to address the shuttle effect (28).
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy provides detailed information about the structure and dynamics of molecules based on the magnetic properties of atomic nuclei. It offers insights into molecular interactions and conformations.
Applications
- Molecular Structure: NMR is useful for elucidating the structures of electrolyte components and analyzing the formation of SEI layers on electrodes.
- Dynamics and Interactions: This technique investigates ion dynamics and molecular interactions within the battery, providing insights into the transport mechanisms and efficiency of ionic conduction.
NMR Research
Solid-state nuclear magnetic resonance (ssNMR) spectroscopy is increasingly recognized for its ability to probe the local structure and dynamics of battery materials. In particular, it has been contributory in the analysis of bulk electrode compounds, electrolytes, and interfaces. While ex situ ssNMR investigations remain prevalent, there has been significant progress in in situ and operando techniques to better understand the reaction mechanisms and degradation processes in electrochemical cells (29). Recent advancements include the development of in situ magic angle spinning (MAS) NMR methodologies, which provide enhanced spectral resolution. This work introduces a mini cylindrical cell insert compatible with 4 mm MAS rotors, demonstrated using a Li/VO2F electrochemical system. The setup allows high-resolution acquisition of 7Li MAS NMR spectra at spinning speeds up to 15 kHz, showcasing its potential for real-time analysis of battery materials under operational conditions (29).
The application of lithium (Li)-7 nuclear magnetic resonance (NMR) spectroscopy for postmortem analysis of lithium metal batteries (LMBs), specifically examining protective-layer coated lithium metal and LiAg alloy electrodes in comparison to bare lithium electrodes was published (30). Utilizing a standard magic angle spinning (MAS) probe, the researchers analyzed 7Li NMR signals to investigate the effects of sample alignment, electrolyte presence, and precycling on the lithium metal surface. The analysis revealed changes in Li0 and Li+ signals before and after cycling, correlating with electrode degradation. The results highlight the effectiveness of protective coatings in mitigating dendrite growth and solid electrolyte interphase (SEI) formation (30). Additionally, magnetic susceptibility anisotropy of Li+ ions near the electrode surface was observed, offering new insights into diamagnetic species characterization on lithium metal electrodes. This study provides practical guidelines for distinguishing lithium states in LMBs and advances the use of 7Li NMR in battery research (30).
Conclusion
The integration of various analytical techniques, including ICP-MS, ICP-OES, μDOES, Raman spectroscopy, XRF, XPS, FT-IR spectroscopy, NIR spectroscopy, UV-Vis spectroscopy, FL, and NMR spectroscopy, is essential in advancing the understanding and development of LIBs. Each method provides unique insights into the composition, structure, and performance of battery materials, ensuring quality and enhancing safety. As the demand for high-performance lithium-ion batteries continues to grow, the ongoing application of these analytical techniques will play a critical role in driving innovation in energy storage technologies. These techniques not only contribute to improved battery performance but also help in the development of safer, more efficient energy storage solutions for a wide range of applications.
Vibrational and associated non-vibrational spectroscopy serves as an indispensable tool in the lifecycle of LIBs, demonstrating the ability to provide detailed insights into the molecular structure and interactions of battery materials to support the continuous innovation and optimization of battery technologies. As the demand for efficient, safe, and high-performance batteries grows, the role of different spectroscopic techniques will undoubtedly expand, contributing to advancements in many energy storage technologies.
LIB Components, Construction, and Technology References
(1) Shen, X.; Zhu, J.; Lai, Q.; Han, Y., BYD Co Ltd, 2012. Lithium Ion Battery. U.S. Patent 8,088,509. https://patents.google.com/patent/US8088509B2/en (accessed 2024-10-16).
(2) Lampe-Onnerud, M. C.; Onnerud, T. P. J., Cadenza Innovation Inc, 2017. Lithium Ion Battery. U.S. Patent 9,685,644. https://patents.google.com/patent/US9685644B2/en (accessed 2024-10-16).
(3) Sandberg, M. G.; Benson, M. R., Delphi Technologies Inc, 2002. Compact Lithium Ion Battery and Method of Manufacturing. U.S. Patent 6,406,815. https://patents.google.com/patent/US6406815B1/en (accessed 2024-10-16).
(4) Chen, G.; Hangzhou Wanma High Energy Battery Co Ltd, 2012. Lithium-Ion Battery. U.S. Patent Application 13/456,198. https://patents.google.com/patent/US20120208050A1/en (accessed 2024-10-16).
(5) Howard, W. G.; Schmidt, C. L.; Scott, E. R. Medtronic Inc, 2010. Lithium-Ion Battery. U.S. Patent 7,807,299. https://patents.google.com/patent/US7807299B2/en (accessed 2024-10-16).
(6) Roberts, M.; Johns, P.; Owen, J.; Brandell, D.; Edstrom, K.; El Enany, G.; Guery, C.; Golodnitsky, D.; Lacey, M.; Lecoeur, C.; Mazor, H. 3D Lithium Ion Batteries—From Fundamentals to Fabrication. J. Mater. Chem. 2011, 21 (27), 9876–9890. DOI: 10.1039/c0jm04396f
(7) Zhang, D.; Tan, C.; Ou, T.; Zhang, S.; Li, L.; Ji, X. Constructing Advanced Electrode Materials for Low-Temperature Lithium-Ion Batteries: A Review. Energy Rep. 2022, 8, 4525–4534. DOI:10.1016/j.egyr.2022.03.130
(8) Deimede, V.; Elmasides, C. Separators for Lithium-Ion Batteries: A Review on the Production Processes and Recent Developments. Energy Technol. 2015, 3 (5), 453–468. DOI: 10.1002/ente.201402215
(9) Tao, T.; Lu, S.; Chen, Y. A Review of Advanced Flexible Lithium-Ion Batteries. Adv. Mater. Technol. 2018, 3 (9), 1700375. DOI: 10.1002/admt.201700375
(10) Ritchie, A.; Howard, W. Recent developments and likely advances in lithium-ion batteries. J. Power Sources 2006, 162 (2), 809–812. DOI: 10.1016/j.jpowsour.2005.07.014
LIB Analytical Method References
(11) Nowak, S.; Winter, M. Elemental Analysis of Lithium Ion Batteries. J. Anal. At. Spectrom. 2017, 32 (10), 1833–1847. DOI: 10.1039/C7JA00073A
(12) Seiffert, S. B.; Riewald, F. F.; Berk, R. B. Single-Particle Elemental Analysis of μm-Sized Battery Materials by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. JES 2024, 171 (5), 050509. DOI: h10.1149/1945-7111/ad4395
(13) Suárez, A.; Jara, A.; Castillo, R.; Gallardo, K. Analysis of Trace Impurities in Lithium Carbonate. ACS Omega 2024, 9 (18), 20129–20134. DOI: 10.1021/acsomega.4c00085
(14) Wiggershaus, B.; Jeskanen, M.; Roos, A.; Vogt, C.;Laurila, T. Trace Element Analysis in Lithium Matrices Using Micro-Discharge Optical Emission Spectroscopy. J. Anal. At. Spectrom. 2024, 39 (5), 1248–1259. DOI: 10.1039/D4JA00044G
(15) Maruyama, S. Operando Raman Observation of Lithium-Ion Battery Graphite Composite Electrodes with Various Densities and Thicknesses. Electrochim. Acta 2024, 498, 144611. DOI: 10.1016/j.electacta.2024.144611
(16) You, E. M.; Gu, Y.; Yi, J.; Wu, D. Y.; Li, J. F.; Tian, Z. Q. Unraveling the Multilayer Solid-Electrolyte Interphase in Lithium Batteries Through Depth-Sensitive Plasmon-Enhanced Raman Spectroscopy: A Theoretical and Experimental Study. Electrochim. Acta 2024, 498, 144689. DOI: 10.1016/j.electacta.2024.144689
(17) Ohno, T.; Padarti, J. K.; Hirai, S.; Matsuda, T. Effects of Coating Layer Homogeneity of Cathode Particles on Lithium Ion Battery Performance. Adv. Powder Technol. 2024, 35 (9), 104608. DOI: 10.1016/j.apt.2024.104608
(18) Petzold, M.; Flamme, S. Recycling Strategies for Spent Consumer Lithium-Ion Batteries. Metals 2024, 14 (2), 151. DOI: 10.3390/met14020151
(19) Wang, J.; Gao, Y.; He, Z.; Feng, X.; Lin, D.; Kong, D.; Zhang, X.; Hu, H.; Guo, Z.; Chen, D. Regulating Structural Units and Morphology of SiOC Anode for Enhanced High-Rate Storage and Long-Life Lithium Ion Batteries. J. Power Sources 2024, 621, 235238. DOI: 10.1016/j.jpowsour.2024.235238
(20) Xu, Y.; Wang, Y.; Chen, X.; Pang, K.; Deng, B.; Han, Z.; Shao, J.; Qian, K.; and Chen, D. Thermal runaway and soot production of lithium-ion batteries: Implications for safety and environmental concerns. Appl. Therm. Eng. 2024, 248, 123193. DOI: 10.1016/j.applthermaleng.2024.123193
(21) Chun, S. Y.; Shim, J. W.; Kwak, K.;Cho, M. Molecular Photothermal Effect on the 2D-IR Spectroscopy of Acetonitrile-Based Li-Ion Battery Electrolytes.J. Phys. Chem. Lett. 2024, 15, (28), 7302–7311. DOI: 10.1021/acs.jpclett.4c00522
(22) Hamidi, S.; Javanbakht, M.; Mousazadeh, M. H.; et al. Forming a Stable SEI Layer by the Synergy Effect of Methyl p-Toluenesulfonate Electrolyte Additive in Li-Ion Batteries. Ionics 2024, 30, 155–167. DOI: 10.1007/s11581-023-05296-1
(23) Ghannoum, A.; Norris, R. C.; Iyer, K.; Zdravkova, L.; Yu, A.; Nieva, P. Optical Characterization of Commercial Lithiated Graphite Battery Electrodes and In Situ Fiber Optic Evanescent Wave Spectroscopy. ACS Appl. Mater. Interfaces 2016, 8, (29), 18763–18769. DOI: 10.1021/acsami.6b03638
(24) Wang, X.; Wang, S. C.; Ma, J.; Xie, R. C.; Wang, W. Near-Infrared Visualisation of Single Microparticle Electrochemistry for Batteries. Chem. Sci. 2024, 15 (22), 8536–8544. DOI: 10.1039/D4SC00072B
(25) Zhou, G.; Sun, X.; Li, Q. H.; Wang, X.; Zhang, J. N.; Yang, W.; Yu, X.; Xiao, R.; Li, H. Mn Ion Dissolution Mechanism for Lithium-Ion Battery With LiMn2O4 Cathode: In Situ Ultraviolet–Visible Spectroscopy and Ab Initio Molecular Dynamics Simulations. J. Phys. Chem. Lett. 2020, 11 (8), 3051–3057. DOI: 10.1021/acs.jpclett.0c00936
(26) Liu, R.; Li, J.; Liu, X.; Yin, X.; Yang, Y. Novel ternary deep eutectic solvents used for recycling lithium and cobalt from waste lithium-ion batteries. Sep. Purif. Technol. 2025, 354, 128934. DOI: 10.1016/j.seppur.2024.128934
(27) Li, X.; Xu, D.; Wang, A.; Peng, C.; Liu, X.; Luo, J. Metal–Organic Cage as Fluorescent Probe for LiPF6 in Lithium Batteries. GEE 2024, 9 (10), 1592–1600. DOI: 10.1016/j.gee.2023.06.001
(28) Coke, K.; Johnson, M. J.; Robinson, J. B.; Rettie, A. J.; Miller, T. S.; Shearing, P. R. Illuminating Polysulfide Distribution in Lithium Sulfur Batteries; Tracking Polysulfide Shuttle Using Operando Optical Fluorescence Microscopy. ACS Appl. Mater. Interfaces 2024, 16 (16), 20329–20340. DOI: 10.1021/acsami.3c14612
(29) Mohammad, I.; Cambaz, M. A.; Samoson, A.; Fichtner, M.; Witter, R. Development of In Situ High Resolution NMR: Proof-of-Principle for a New (Spinning) Cylindrical Mini-Pellet Approach Applied to a Lithium Ion Battery. Solid State Nucl. Magn. Reson. 2024, 129, 101914. DOI: 10.1016/j.ssnmr.2023.101914
(30) Baek, J.; Kim, S.; Kim, H. T.; Han, O. H. Postmortem 7Li NMR Analysis for Assessing the Reversibility of Lithium Metal Electrodes in Lithium Metal Batteries. J. Energy Chem. 2024, 94, 430–440. DOI: 10.1016/j.jechem.2024.02.063
Jerome Workman, Jr. serves on the Editorial Advisory Board of Spectroscopy and is the Senior Technical Editor for LCGC and Spectroscopy. He is the co-host of the Analytically Speaking podcast and has published multiple reference text volumes, including the three-volume Academic Press Handbook of Organic Compounds, the five-volume The Concise Handbook of Analytical Spectroscopy, the 2nd edition of Practical Guide and Spectral Atlas for Interpretive Near-Infrared Spectroscopy, the 2nd edition of Chemometrics in Spectroscopy, and the 4th edition of The Handbook of Near-Infrared Analysis. JWorkman@MJHlifesciences.com●


LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.