Analysis of Yeast Fermentation Using Multiple Reflection Diamond FT–IR ATR
Application Notebook
Fermentation processes have been used since the Neolithic era and are widely used today in diverse fields such as foods, beverages, and pharmaceuticals.
Fermentation processes have been used since the Neolithic era and are widely used today in diverse fields such as foods, beverages, and pharmaceuticals. Hence, there is a continuing need for more effective and faster methods of analyzing the chemistry behind the fermentation process.
This applications note explores the potential of using multiple-reflection diamond ATR to examine the yeast-mediated conversion of sucrose into ethanol. Both sucrose and ethanol exhibit strong absorption bands in the mid-infrared spectral region, and thus ATR can be used to track the progress of the fermentation process.
Experimental
Two types of Lalvin yeast were examined: EC1118 Dry Wine Champagne Yeast and ICV-D47 Yeast. For each, 1.5 g of sugar was dissolved in 50 mL tap water and then 1.5 g of yeast was added. The mixtures were stirred every 15 min and were covered in the interim to minimize evaporation. All samples were kept at ambient temperature.
Infrared spectra were collected on an FT-IR spectrometer equipped with the Harrick ConcentratIR2™ multiple-reflection diamond ATR accessory (Figure 1). The ConcentratIR2 is able to measure analytes having very low concentration in aqueous or other liquid solutions, and requires only a very small sample volume of ~10 μL. The system was purged to remove water vapor and CO2. Spectra were collected at 4 cm-1 resolution and signal averaged over 128 scans. A background was collected with the accessory in the sample compartment and no sample on the crystal. Then a drop of sample was placed on the ATR crystal and the spectrum was collected. Spectra were collected over the first 3 h with an additional spectrum recorded ~24 h after fermentation began. The spectrum of water, collected under the same conditions, was subtracted from each spectrum.

Figure 1: ConcentratIR2™ Multiple Reflection ATR.
Results and Discussion
The resulting spectra are shown in Figure 2. The set of overlapping bands from approximately 1200–950 cm-1 correspond to sucrose. Ethanol has two bands in the same range, which are initially obscured by the sucrose bands but emerge with decreasing sugar concentration. Looking at the changes over time, it is clear that the EC1118 yeast ferments more quickly than the ICV-D47, as expected from its rating (1). This demonstrates that it is possible to observe the fermentation process with multiple reflection diamond ATR. A more detailed analysis could be performed using partial-least squares (PLS) multivariate regression method to predict the concentrations of sucrose and ethanol. Flow-through and heated cells are available with the ConcentratIR2 to facilitate kinetics studies.
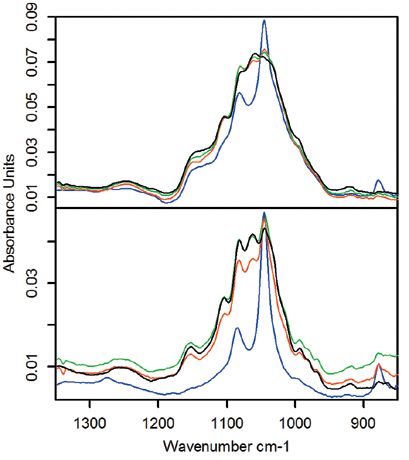
Figure 2: Infrared spectra of ICV-D47 (top) and EC1118 (bottom) yeast fermentation. Black: 1 h, green: 2 h, red: 3 h, blue: 24 h.
Conclusion
Multiple reflection diamond ATR spectroscopy is a valuable method for analyzing fermentation and other aqueous reactions. Yeast fermentation in an aqueous sucrose solution was observed using the Harrick ConcentratIR2™ ATR accessory. It was immediately possible to see relative rates between the different yeasts. Further analysis is possible to obtain more rigorous quantitative data or kinetics data to derive reaction rates.
References
(1) Scott's Laboratories 2013 Fermentation Handbook, 8-11.
Harrick Scientific Products, Inc.
141 Tompkins Ave., 2nd Floor, Pleasantville, NY 10570
tel. (800) 248-3847
Website: www.harricksci.com

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.