Benefits of ICP-MS for the Elemental Compositional Analysis of Lithium-Ion Battery Electrolytes
This month’s column investigates the elemental composition of electrolytes in lithium-ion batteries (LIBs) using inductively coupled plasma–mass spectrometry (ICP-MS). In addition to offering quantitative results for the target elements, the technique also provided valuable insight into the semiquantitative analysis of up to 78 elements in the samples. To complete the study, method detection limits and long-term stability in LIB electrolyte matrices were also evaluated.
The development and widespread adoption of clean energy technologies, such as electric vehicles and renewable energy storage systems powered by lithium-ion batteries (LIBs), are central to achieving global decarbonization goals (1–3). However, trace-elemental impurities in LIBs have been well-documented to negatively impact their quality, performance, safety, and operational lifespan (4). To address these challenges, accurate and robust testing methods, particularly those based on atomic spectroscopic techniques like inductively coupled plasma–mass spectrometry (ICP-MS), play an important role in monitoring the elemental profiles of LIB-related materials. ICP-MS has proven indispensable for analyzing samples across all stages of LIB production, from raw materials and chemical components—such as cathodes, anodes, and electrolytes—to degraded and recycled materials (5).
Currently, most commercial LIBs use a liquid electrolyte comprising a mix of lithium electrolyte salts, such as lithium hexa-fluorophosphate (LiPF6), lithium tetrafluoroborate (LiBF4), lithium perchlorate (LiClO4), and additives, dissolved in organic solvents (6–8). The solvents are normally composed of proprietary mixtures of high-purity cyclic and chain carbonates including dimethyl carbonate (DMC), ethyl methyl carbonate (EMC), and ethylene carbonate (EC) (8,9). The solvents make the charge carrier lithium (Li) ions mobile, so they can migrate between the cathode and anode during the battery’s charge and discharge cycles.
Elemental impurities and contaminants such as chromium (Cr), iron (Fe), nickel (Ni), copper (Cu), and zinc (Zn) in the electrolyte solvent (and other components of a LIB) can have a significant impact on the performance and safety of the battery. Adverse effects can include changes to ion mobility, undesired electrochemical reactions, gas evolution, and other hazards such as Li dendrite growth (4). These effects can also result from changes in the electrolyte composition caused by repeated charge cycles over time (4).
Various complementary analytical techniques are required to establish a complete profile of the organic and inorganic composition of electrolytes, whether for degradation monitoring or for investigating the formulation of commercial electrolytes (10).
In this study, we used ICP-MS to analyze the inorganic formulation of three unknown commercial electrolytes, following an initial compositional analysis of the organic components and additives using other mass spectrometry technologies.
Experimental
Samples and Sample Preparation
Three different liquid electrolyte samples were obtained from a commercial LIB manufacturer in Southeast Asia. No special sample preparation was implemented for the samples except solvent dilution. Lithium electrolytic salts pose challenges for ICP-MS analysis because of the significant solvent-carbon and salt effects (11). Based on an initial compositional analysis by other techniques, DMC was identified as the main component in all three samples with a minimal amount of EMC and EC (10). To avoid unexpected contaminants from additional solvents and to minimize the matrix effects because of electrolytic salts, the electrolyte samples were diluted 100 times using high-purity, battery-grade DMC (≥99% purity on a trace metals basis, Sigma-Aldrich). The carbon enhancement effect can be compensated for by preparing the calibration standards in a matrix that closely matches the sample composition (matrix-matching) or by spiking standards into a representative sample, a calibration approach known as standard addition.
Calibration
A 21-element organometallic standard solution in hydrocarbon oil (Agilent Technologies) was used as the stock to prepare matrix-matched calibrations in a customized solvent mixture of DMC/EMC (70/30 v/v) to simulate the electrolyte sample matrix. The EMC was ≥99% purity on a trace metals basis (Sigma-Aldrich). The stock standard contained Ag, Al, B, Ba, Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, Sn, Ti, V, and Zn at a concentration of 500 µg/g (ppm). A 5-ppm intermediate standard was prepared by taking 20 µL of the 500-ppm stock standard solution and diluting it to 2 mL total volume with the solvent mix. This intermediate standard mix was then used to prepare the spikes for the calibration standards in the DMC/EMC solvent. Nine standards were prepared by spiking an appropriate amount of the intermediate (5 ppm) standard into the solvent mix to give spike levels between 1.0 and 500 ppb for all 21 elements. The unspiked solvent was used as the calibration blank.
Instrumentation and Methodology
The elemental analysis of the electrolytes was performed using a 7900 ICP-MS (Agilent Technologies), which was configured for organic sample analysis and controlled using the instrument software (MassHunter, Agilent Technologies). Knowing that the main constituent of the unknown samples was DMC, the instrument conditions and parameters were optimized based on a previous study (12). The ICP-MS instrument was equipped with an organic solvent sample introduction kit and platinum-tipped interface cones. All samples were introduced via an autosampler. Other added instrument components included a MicroMist nebulizer, a quartz torch with 1.5-mm internal diameter (id) injector, an integrated autosampler probe (0.15 mm i.d.), solvent-resistant uptake and drain tubing (all equipment Agilent Technologies). The optional fifth gas line for the addition of a 20/80 oxygen/argon (O2/Ar) mix to the plasma was fitted to prevent the deposition of elemental carbon (soot) on the interface cones.
The ICP-MS system also includes a fourth-generation collision reaction cell (CRC), that can operate in helium (He) collision mode and high energy He collision mode (HEHe), or with a reactive cell gas to control spectral interferences that arise from the organic matrix. This capability reduces the impact of many polyatomic ion overlaps, which enables the collection of full mass range screening data for up to 78 elements in a few seconds using IntelliQuant (Agilent Technologies), making it ideal for the elemental profiling of complex sample matrices (13). This approach was used to gain greater insights into the composition of the real-world electrolyte samples.
ICP-MS instrument operating parameters are given in Table I.
Results and Discussion
Elemental Profiling
A quick, full mass “all-element” scan of the three unknown electrolytes was run in He collision mode, providing an overview of the elemental profile of each sample and helping to identify which elements to study further. The quick scan data was processed using the IntelliQuant function within the instrument software. Figure 1 shows the semiquantitative data displayed in a periodic table heat map view of all three samples. The elements with a darker red color, such as Li, B, P, and Cl, indicate the relatively higher concentrations present in the electrolytes. Other elements were also observed as impurities, including (but not limited to) Na, K, Mg, Cr, Fe, Zn, and Ni. Therefore, a further study using a fully quantitative based on a matrix-matched (DMC/EMC solvent) external calibration was carried out to quantify many of these elements in the samples. The quick scan data also helped identify the optimum calibration range for the 21 analytes of interest.
FIGURE 1: Periodic table heat map display of semiquantitative data obtained for three electrolyte samples (Sample (a) 1, (b) 2, (c) 3) providing a visual overview of the elemental profile of the samples.

Calibration and Sensitivity
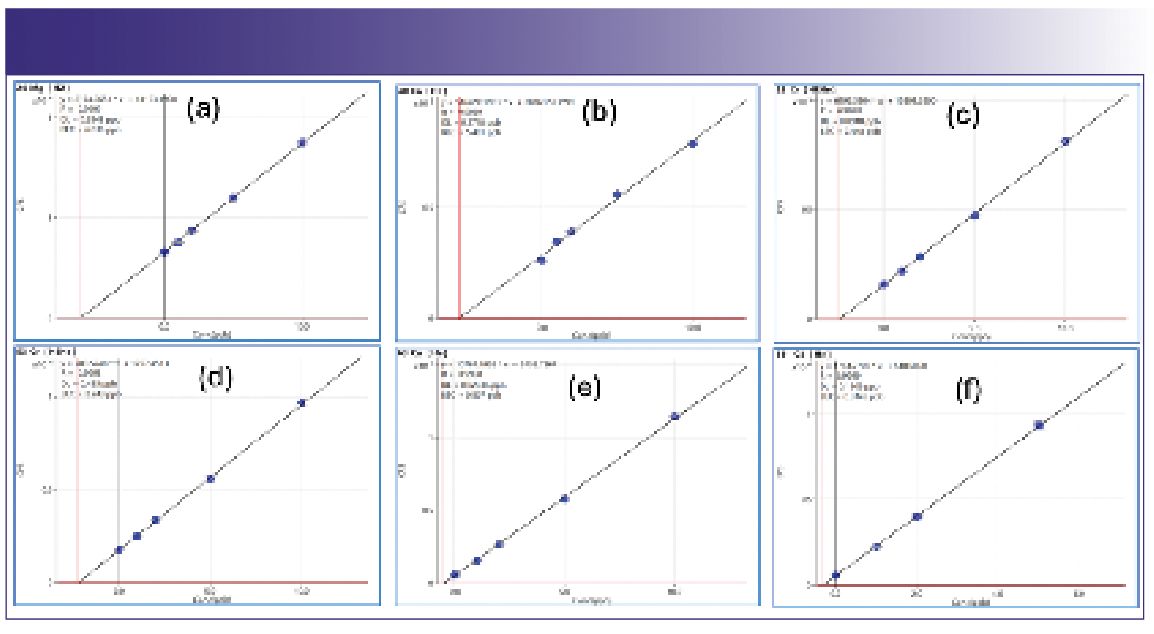
Representative calibration curves for several interfered elements, 24Mg, 40Ca, 52Cr, 53Cr, 63Cu, and 111Cd, are shown in Figure 2. The reproducible and consistent linearity of the curves demonstrates the effectiveness of various CRC conditions for the accurate determination of these elements in the DMC/EMC solvent matrix. He collision mode was used as the default setting for most analytes, while H2 reaction mode was used to attenuate the polyatomic interference from 12C2 on 24Mg and the isobaric interference from 40Ar on 40Ca. The very similar calibration curves obtained for 52Cr and 53Cr in HEHe collision mode illustrates the effectiveness of the CRC in removing the severe ArC+ polyatomic ion overlap at mass 52. The Cr comparison also demonstrates the potential use of multiple isotopes (where available) to provide an internal qualifier measurement to confirm the results. Alternative cell gas modes can be used in a similar way. The linearity and accurate analysis at low, sub-ppb levels for Cu and Cd demonstrate the benefit of ICP-MS for ultra trace-level contamination control of high-purity LIB electrolyte solvents.
FIGURE 2: Matrix-matched calibration plots for (a) 24Mg, (b) 40Ca (H2 reaction mode), (c) 52Cr and (d) 53Cr (HEHe mode), (e) 63Cu, and (f) 111Cd (He collision mode) in DMC/EMC, showing spike levels up to 5 or 10 μg/L (ppb).
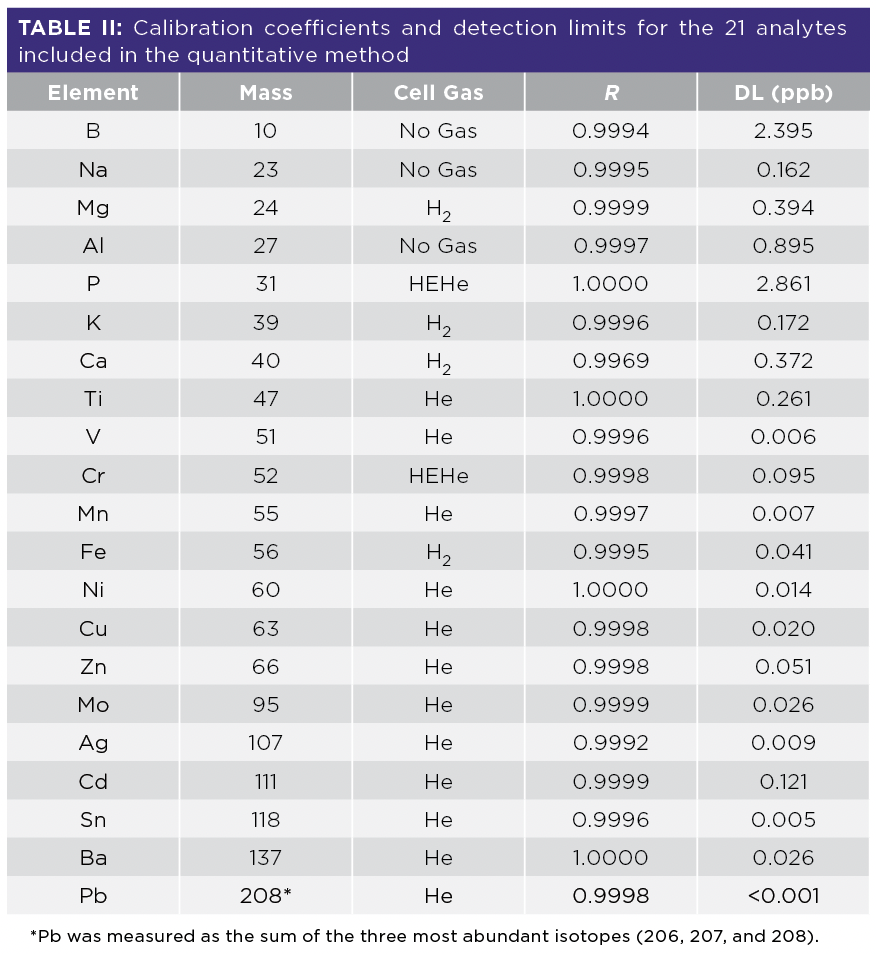
Calibration and detection limit data for all 21 analytes is given in Table II, confirming the method’s sensitivity and suitability for the accurate analysis of a range of elements in LIB electrolytes.
Quantification
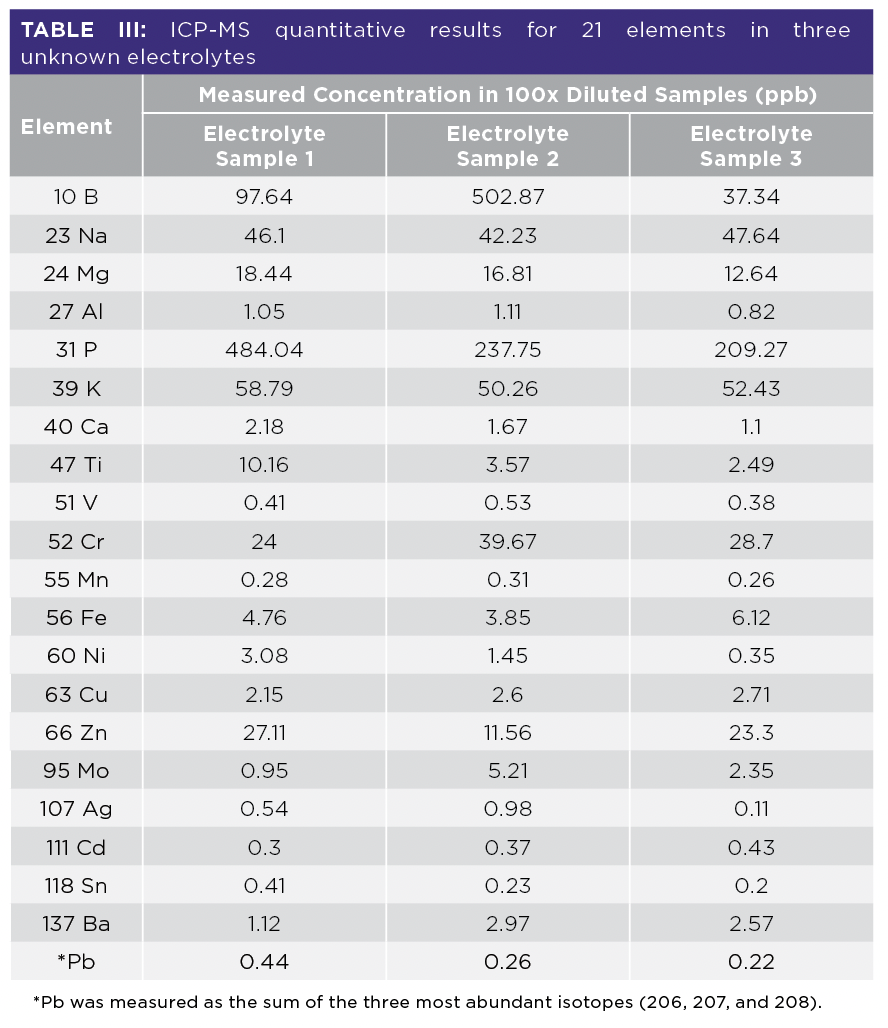
The quantitative results for the selected 21 elements measured in the three unknown electrolyte samples are summarized in Table III. These results, when viewed together with the semiquantitative data, indicate that lithium salts, LiPF6, LiBF4, and LiClO4, are prevalent in the three electrolyte samples with varying ratios of each salt. These insights provide valuable information about the formulation of Li salts in unknown liquid electrolytes.
Long-Term Stability
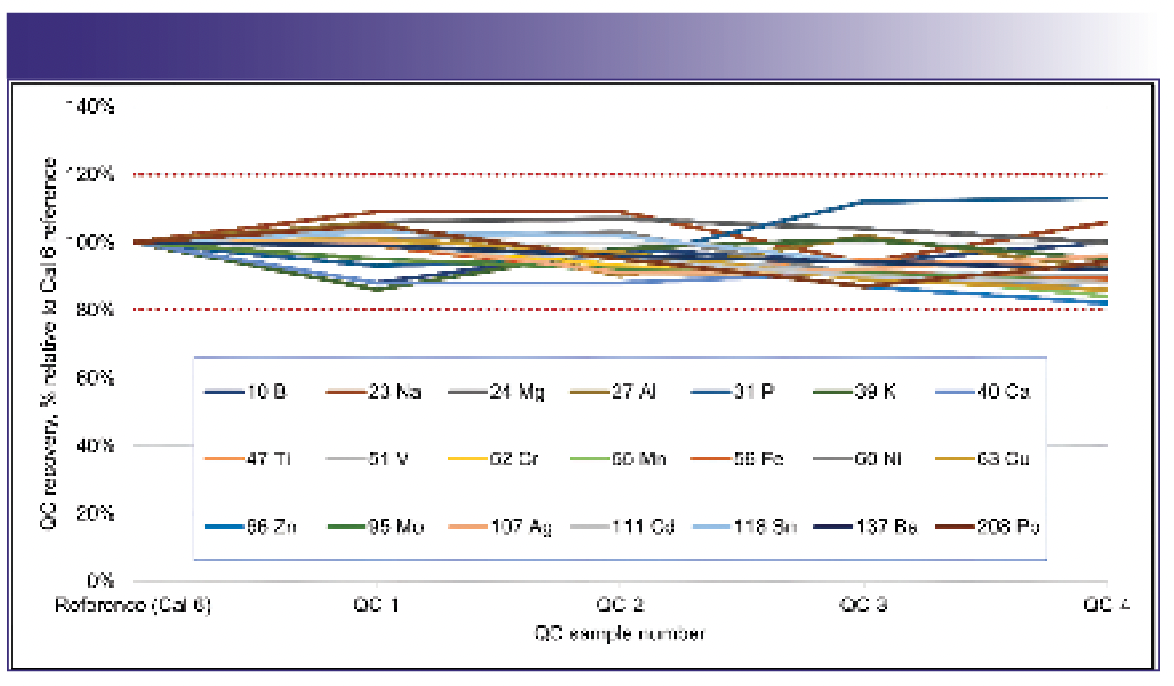
A long-term stability test of the ICP-MS instrument was performed to check its suitability for the routine analysis of LIB electrolytes over a 6-h continuous analytical run. A quality control solution (QC) was prepared from a mix of DMC/EMC spiked at 20 ppb with the 21 target elements. The QC sample was run after every 10 samples. Figure 3 shows the normalized QC recoveries (%) for all the analytes over the course of the analysis, using the initial 20 ppb calibration spike as the reference. Recoveries were all within ±20% (between 80 and 115%) throughout the six-hour run, with good precision (less than 10% RSD). The stability of the QC sample demonstrates the high matrix tolerance of the ICP-MS system and confirms the ability of the CRC to control the matrix-based interferences on analytes such as Mg, P, and Cr.
FIGURE 3: QC recoveries for a 20-ppb spike in the DMC/EMC matrix run after every 10 samples during a six-hour analytical sequence.
Conclusion
An ICP-MS instrument configured for organic solvent analysis identified the elemental composition of three unknown electrolyte solutions, complementing the analysis of volatile and non-volatile organic constituents by GC–MS and LC–QTOF-MS. By controlling carbon-based interferences, a complete overview of the elemental content of the samples was obtained through the acquisition of both quick scan (semiquantitative) and quantitative data. Researchers can combine the data sets from multiple techniques to monitor electrolyte degradation over time or to investigate the composition of uncharacterized samples. The ICP-MS data indicated the presence of LiPF6, LiBF4, and LiClO4 in the three electrolyte samples.
The ICP-MS method is also suitable for the routine quality control analysis of inorganic impurities in solvent-based electrolytes—and other LIB-related samples—at low and sub-ppb levels. Ensuring the quality of components and materials used in LIB manufacturing is important for enhancing the performance of both current and next-generation batteries.
References
- Kamat, P.V. Lithium-ion batteries and beyond: celebrating the 2019 Nobel prize in chemistry - a virtual issue. ACS Energy Lett., 2019, 4 (11), 2757-2759. DOI: 10.1021/acsenergylett.9b02280
- Wrublewski, D.T. Analysis for science librarians of the 2019 Nobel prize in chemistry: lithium-ion batteries. Sci. Technol. Libr. 2020, 39 (1), 51-67. DOI: 10.1080/0194262X.2020.1717405
- Korthauer, R. Lithium-Ion Batteries: Basics and Applications, Springer Berlin, Heidelberg, 2018, https://link.springer.com/book/10.1007/978-3-662-53071-9
- Blomgren, G. E. The Development and Future of Lithium-Ion Batteries. J Electrochem Soc. 2017, 164, A5019. DOI: 10.1149/2.0251701jes/pdf
- Workman, J. A Comprehensive Review of Spectroscopic Techniques for Lithium-Ion Battery Analysis. Spectroscopy 2024, 39, s11,6–16. https://doi.org/10.56530/spectroscopy.ii3689u3
- Zhang, S. S.; et al. LiPF6–EC–EMC electrolyte for Li-ion battery. J. Power Sour. 2002, 107, 18-23. DOI:10.1016/S0378-7753(01)00968-5
- Qin, N.; et al. Trace LiBF4 Enabling Robust LiF-Rich Interphase for Durable Low-Temperature Lithium-Ion Pouch Cells. ACS Energy Lett. 2024, 9 (10), 4843–4851. DOI: 10.1021/acsenergylett.4c01616
- Bolloju, S.; et al. Electrolyte additives for Li-ion batteries: classification by elements. Prog. Mat. Sci. 2025, 147, 10139. DOI: 10.1016/j.pmatsci.2024.101349
- Kainat, S.; et al. Electrolytes in Lithium-Ion Batteries: Advancements in the Era of Twenties (2020's). Mater. Chem. Phys. 2024, 313, 128796. DOI: 10.1016/j.matchemphys.2023.128796
- Zou, A. M.; et al. Multiplatform Approach for Lithium-Ion Battery Electrolyte Compositional Analysis. Agilent Technologies application note, 2024, 5994-7014EN
- Liu, S.; et al. Organic matrix effects in inductively coupled plasma mass spectrometry: A tutorial review. Appl. Spectrosc. Rev. 2022, (6), 461–489. DOI: 10.1080/05704928.2021.1897991
- Zou, A. M., et al., Analysis of Elemental Impurities in Lithium-Ion Battery Electrolyte Solvents by ICP-MS, Agilent Technologies application note, 2023, 5994-6883EN
- McCurdy, E.; Riles, P. Non-Specific Calibration Combined with Helium Collision Mode for Elemental Screening. Spectroscopy 2023, 38 (8), 26–28. DOI: doi.org/10.56530/spectroscopy.ld6080l6
About the Column Editor
Robert (Rob) Thomas is the principal scientist of Scientific Solutions, a consulting company that serves the educational needs of the trace element user community. He has worked in the field of atomic and mass spectroscopy for more than 50 years, including 24 years for a manufacturer of atomic spectroscopic instrumentation. Rob has written over 100 scientific publications, including six textbooks on the fundamental principles and applications of ICP-MS.
About the Author
Aimei Zou is a Solution Scientist at Agilent Technologies with over 16 years of expertise in method development, specializing in atomic spectroscopy and mass spectrometry.


LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Atomic Perspectives: Highlights from Recent Columns
March 3rd 2025“Atomic Perspectives,” provides tutorials and updates on new analytical atomic spectroscopy techniques in a broad range of applications, including environmental analysis, food and beverage analysis, and space exploration, to name a few. Here, we present a compilation of some of the most popular columns.