Bottled Water: Popular, But Is It Safe?
Special Issues
Bottled water has become increasingly popular over the past several years for convenience and safety. In some areas where publicly supplied tap water is contaminated or contains bacteria, this assumption is valid. However, in areas with clean tap water, the presence of bottled water can be controversial because it might be less clean than the local tap. This article discusses the analysis of inorganic contaminants in bottled water, including regulated contaminants and bromate. Detection limit considerations and speed of analysis also are discussed.
Drinking water is essential for life and, in its most desirable form, is free of bacteria and chemical contaminants. Often, we assume that bottled water is cleaner than tap water, and in some parts of the world, that is certainly the case. Bottled water is popular in the U.S., outselling milk for the first time in 2006 (1). The largest consumer of bottled water (per capita) is Italy, hardly a developing nation (2). In the U.S., bottled water and drinking water contaminant regulations have been harmonized, so that both types of water should be of the same quality. However, in places with inadequate regulations or regulations that are not well enforced, bottled water can be more contaminated than expected.
Water testing is critical to ensuring quality. Even though standards have been set and implemented to ensure the initial quality for a water, the bottling process can have unexpected consequences. For example, a famous bottled water was recalled after benzene was unexpectedly detected (3). A recent report found that 10% of bottled water in Shanghai, China, does not meet sanitary standards (4). Earlier this year, bottled water from Armenia was recalled because of contamination with more than 50 times the Food and Drug Administration–regulated amount of arsenic (5). Water is analyzed for three broad classes of contaminants: microbiological, organic, and inorganic. Inorganic contaminants include metals such as lead (Pb) and cadmium (Cd), that are clearly toxic. Some elements, such as selenium (Se), might be beneficial at low levels but toxic at higher concentrations. U.S.-regulated primary and secondary drinking water inorganic contaminant levels are shown in Table I.
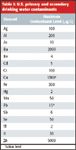
Table I: U.S. primary and secondary drinking water contaminants
Metals Analysis Helps Ensure Safety
Bottled water from around the world has been collected and analyzed to compare the inorganic contaminants to those allowed in the U.S. Several analytical techniques are suitable for the measurements, and in this case, inductively coupled plasma-mass spectrometry (ICP-MS) was chosen for speed and detection level, using Environmental Protection Agency (EPA) method 200.8 (6). Detection limits 10 times below the concentration at which decision-making is required generally are recommended. Consensus methods and U.S. EPA methods generally require a number of quality control tests interspersed with samples for analysis to ensure high-quality data results for these important measurements.
Table II shows a variety of bottled water results. The results are below the regulatory limit in all cases. For many elements, the concentration is below the detection limit as measured using the EPA definition. For several bottled water samples, it is interesting to note the presence of uranium. Although it is below the regulatory limit, it is clearly present and can be detected at very low levels by ICP-MS.

Table II: Drinking water contaminants measured in a variety of bottled waters (μg/L) using the PerkinElmer Sciex ELAN DRC II ICP-MS
Speciation Distinguishes Metallic Forms
Disinfection with ozone rather than chlorine is becoming more popular for bottled water because it does not leave a residual smell or taste. Because bottled water is sealed after production, there is less chance that recontamination with bacteria will take place than in piped delivery systems. Because bromide (Br–) is present naturally in some water, ozone disinfection will promote the formation of bromate (BrO3–), a carcinogen. Although total bromine can be measured with ICP-MS, it is not possible to tell how much of the bromine is present in the bromate form. Inorganic speciation is a growing area of analysis in which a separation of the various forms of the same element takes place before the elemental measurement. Several of the bottled waters reported earlier also were analyzed for bromate using ICP-MS coupled with high performance liquid chromatography (HPLC), which separated the Br– and BrO3– before the metal determination. The U.S. and European regulated bromate level is 10 μg/L (ppb).
Recent Advances in Bromate Measurement
Since the development of U.S. EPA method 321.8 in 1998, advances in speciation have taken place, allowing the method to be improved for ruggedness and speed. Figure 1 shows a chromatogram of 1 μg/L of bromate, showing that even at very low concentrations, it can be distinguished from the background levels (7).
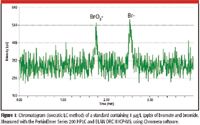
Figure 1: Chromatogram (isocratic LC method) of a standard containing 1 μg/L (ppb) of bromate and bromide. Measured with the PerkinElmer Series 200 HPLC and ELAN DRC II ICP-MS, using Chromera software.
Note that time for analysis is less than 3 min, whereas the method 321.8 chromatographic separation takes 8 min. Figure 2 shows overlaid chromatograms of a Chinese bottled water analyzed 49 times over the course of several hours to examine the short-term precision, which is better than 2%.
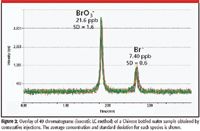
Figure 2: Overlay of 49 chromatograms (isocratic LC method) of a Chinese bottled water sample obtained by consecutive injections. The average concentration and standard deviation for each species is shown.
For a few samples with asymmetrical peaks and high bromate values, additional method development was done to ensure that the analyte of interest was separated adequately from any other components that might mistakenly contribute to the bromate measurement. A gradient HPLC method, although slightly longer than the isocratic method developed for routine use, showed that several peaks overlapped the bromate peak in some samples (8). Advanced interference correction on the ICP-MS system showed that the additional peaks did, in fact, contain bromine. Their identification was not investigated further. Figure 3 shows the chromatogram for bottled water purchased in Thailand showing the additional peak, uncovered with the gradient separation method. Table III shows the concentrations of bromide and bromate for a variety of bottled and tap water samples. The samples with suspect peaks or bromate concentrations above the regulatory limit were confirmed with the developed gradient HPLC separation to ensure that only one peak was present at the retention time expected for bromate.

Figure 3: Chromatogram of a bottled water from Thailand with (a) an isocratic HPLC separation method and (b) a gradient HPLC separation method.
Speciation of Other Elements
Although few elements are regulated by their species, there are many whose form influences their toxicity. For example, chromium VI is known to be much more hazardous than chromium III. So in the future, as inorganic speciated analysis becomes more common, it is likely that measurements will yield more information on water toxicity than at present. Figure 4 shows chromatograms collected simultaneously for four elements and their species. Because ICP-MS is a rapid multielement detector, separation of several elements at once takes advantage of its multielement capability and provides a more efficient analysis (9).

Table III: Quantitative determination of bromide and bromate in bottled and tap water samples confirmed with a gradient HPLC method (all units in μg/L)
Conclusions
Water analysis is critical to a safe and compliant bottled water supply. Measuring inorganic components is an important part of the complete analysis and can be performed efficiently using ICP-MS. Measurements should be performed on a regular basis to account for any changes that might have occurred at the source or in the bottling process.
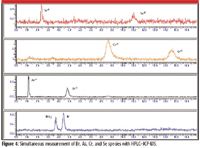
Figure 4: Simultaneous measurement of Br, As, Cr, and Se species with HPLCâICP-MS.
Speciation measurements are a more recent type of analysis that couples two analytical techniques for the separation and detection of inorganic components. Speciation can be used right now for the determination of the disinfection byproduct bromate and might be further applied in the future as the species of other elements, such as arsenic or selenium, become regulated.
Zoe A. Grosser, Lee Davidowski, and Kenneth R. Neubauer are with PerkinElmer Life and Analytical Sciences, Shelton, Connecticut.
References
(1) Water Industry News, May 4, 2007, http://www.watertechonline.com/news.asp?N_ID=67248.
(2) The Globalist, July 9, 2006.
(3) George James, New York Times, February 10, 1990.
(4) Water Technology On-line, June 27, 2006.
(5) Water Technology On-line, March 27, 2007.
(6) R. Wolf, E. Denoyer, and Z. Grosser, EPA Method 200.8 for the Analysis of Drinking Waters, PerkinElmer Application Note D-6527, 2001.
(7) K.R. Neubauer, W.M. Reuter, P.A. Perrone, and Z.A. Grosser, Bromate/Bromide Speciation by HPLC-ICP-MS, presentation at PITTCON, Orlando, Florida, March 2006.
(8) K.R. Neubauer, W.M. Reuter, and P.A. Perrone, Application Note 007303A_01, PerkinElmer Corporation, Shelton, Connecticut (2006).
(9) K.R. Neubauer, W.M. Reuter, P.A. Perrone, and Z.A. Grosser, Application Note 007050_01, PerkinElmer Corporation, Shelton, Connecticut (2004).

Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.
Best of the Week: Hyperspectral Imaging, ICP-MS Analysis of Geological Samples, Product Roundup
October 18th 2024Top articles published this week include an article about hyperspectral imaging in human skin research, a peer-reviewed article about analyzing geological samples using atomic spectroscopy techniques, and an equipment roundup piece about the latest products in the industry.










