Analysis of the Brill Transition and Reversible Brill Transition in Nylon 6,6 by Raman Spectroscopy
Spectroscopy
This article verified the Brill transition in nylon 6,6 by Raman spectroscopy through heating and cooling processes of the sample. When nylon is heated at around 160 C a crystalline phase transition occurs from a triclinic structure at room temperature to a pseudohexagonal structure above that temperature. This phase transition is known as the Brill Transition. With temperature-dependent Raman scattering measurements, it was possible to determine the vibrational behavior of nylon 6,6 during the Brill transition, and consequently to identify the main Raman bands associated with the Brill transition.
This article describes the analysis of the Brill transition in nylon 6,6 by Raman spectroscopy through heating and cooling processes of the sample. When nylon is heated at approximately 160 °C, a crystalline phase transition occurs from a triclinic structure at room temperature to a pseudohexagonal structure above that temperature. This phase transition is known as the Brill transition. With temperature-dependent Raman scattering measurements, it was possible to determine the vibrational behavior of nylon 6,6 during the Brill transition, and consequently to identify the main Raman bands associated with the Brill transition.
It is part of the nature of semicrystalline polymers to present several relaxations in the polymeric structure. These relaxations are associated with the mobility of the chain segments in the crystalline and amorphous regions of the polymer, which lead to changes in the physical properties because of the interactions between crystalline and amorphous regions (1–3). In 1942, R.J. Brill (4) first reported what we today know as the Brill transition in nylon 6,6. Today we know that during the Brill transition in nylon 6,6, the triclinic crystal structure at room temperature is gradually replaced by a different triclinic structure at the Brill transition temperature (TB), this high-temperature crystalline form was characterized as a pseudohexagonal phase (4–7). The TB of nylon 6,6 has been determined to be between 160 °C and 180 °C by several techniques, including differential scanning calorimetry (8,9) and nuclear magnetic resonance (NMR) (2,10). However, the nature of the Brill transition is still not completely understood (11), since it occurs over a broad temperature range from 120 °C to 180 °C. We performed Raman spectroscopic investigations of the Brill transition and, moreover, verified its reversibility, since this has not been previously done via Raman spectroscopy for this purpose.
Materials and Methods
Nylon 6,6 was acquired from Sigma-Aldrich: poly[imino(1,6-dioxohexamethylene) iminohexamethylene] (nylon 6,6) [-CO(CH2)4CONH(CH2)6NH-]n, with molecular weight Mw = 262,35 g/mol and density d = 1.09 g/mL.
All the Raman spectra of this polymer were acquired with a Thermo Scientific DXR Raman microscope, equipped with an Olympus BX41 confocal microscope. The spectrometer system was operated with the Thermo Omnic acquisition software.
A continuous wave (CW) laser with an excitation wavelength of 532 nm (2.33 eV) was used. Magnification with a 10x objective (NA = 0.25) yielded a laser spot diameter of 2.5 µm. The laser power on the sample was 9.0 mW, resulting in an irradiance of 1.8 µW µm-2. Next, 15 accumulations were collected with an exposure time of 10.0 s each for all spectra, both in the spectral range of 100–3500 cm-1 with a spectral resolution of 5.0 cm-1 (full range) and in the spectral range of 100–1850 cm-1 with a spectral resolution of 2.0 cm-1 (high resolution). For all Raman spectra an automatic baseline correction by means of the Thermo Omnic acquisition software was performed.
The temperature of the polymeric sample was controlled via a T95 system and a cooling option LNP95, both from Linkam Scientific Instruments Ltd. A heating rate of 20 °C/min, and a temperature step size of 10.0 °C, with a precision of ±0.1 °C, were selected in all cases. The sample was measured without any physical or chemical pretreatment.
Results and Discussion
We carefully analyzed all Raman peaks of the nylon 6,6 sample as a function of temperature. The peaks at 1126 cm-1 and 1479 cm-1 shown in Figure 1 (obtained from the full range spectra) display substantial changes in their intensities between 40 °C and 200 °C. It is possible to recognize in Figure 1 that these peaks at 1126 cm-1 and 1479 cm-1 disappear between 160 °C and 180 °C.
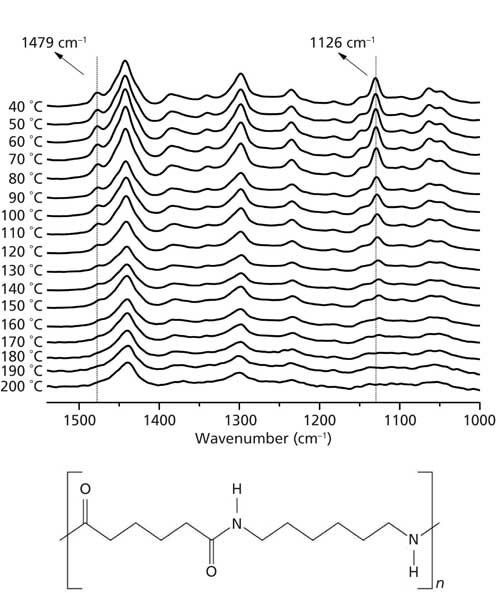
Figure 1: Raman spectra of nylon 6,6 collected as a function of temperature from 40 °C to 200 °C in the wavenumber range from 1000 cm-1 to 1550 cm-1 (“full range”). The highlighted band at 1126 cm-1 is due to a C-C skeletal stretching mode and the band at 1479 cm-1 is due to the C-N and N-H bending modes. With rising temperature these bands disappear above 170 °C. The structure of nylon 6,6 is also shown.
The same behavior can be observed in Figure 2a, collected at “high resolution” in the temperature range from 120 °C to 200 °C. The behavior of these bands confirms that nylon 6,6 suffers some structural changes between 160 °C and 180 °C. TB falls exactly in this range of temperatures (11), thus these peaks can be named Brill Raman bands. It has been reported that the disappearance of these bands may be because of a local “melting” of the methylene segments (7). It corroborates the fact that the peak centered at 1479 cm-1 is admittedly associated to the vibrational bending mode of C-N and N-H bending (12). Therefore, the local “melting” process can free the librational motion of the methylene segments, justifying thus the disappearance of this peak in Raman spectra during the Brill transition (7).
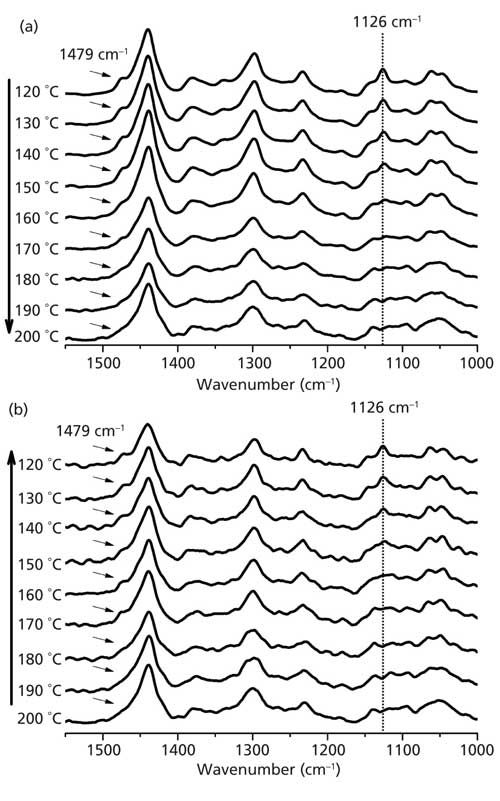
Figure 2: Raman spectra of nylon 6,6 collected in the wavenumber range from 1000 cm-1 to 1550 cm-1 (“high resolution”) as a function of temperature (a) for increasing temperatures from 120 °C to 200 °C and (b) for decreasing temperatures from 200 °C to 120 °C. The highlighted band at 1126 cm-1 is due to a C-C skeletal stretching mode, and the band at 1479 cm-1 is due to C-N and N-H bending modes.
In sequence, we also ran a cooling procedure with nylon 6,6 from 200 °C to 120 °C and collected the Raman spectra at high resolution. The results are shown in Figure 2b, where it is possible to see the reversibility of the process, since the Brill Raman bands emerge again below 180 °C.
Because of the more symmetric shape of the peak at 1126 cm-1, which is associated with C-C skeletal stretching vibration (12), we fitted this peak using a Gaussian function. In the case of the full range Raman spectra, the peak area as a measure of the Raman intensity is shown in Figure 3 as a function of temperature in the range from 40 °C to 200 °C. One can recognize a decreasing peak intensity with rising temperature; above 170 °C, this intensity goes to zero, showing that this band at 1126 cm-1 disappears. This finding by Raman spectroscopy is in agreement with the observation by NMR measurements of structural changes during the Brill transition in nylon 6,6 (13).
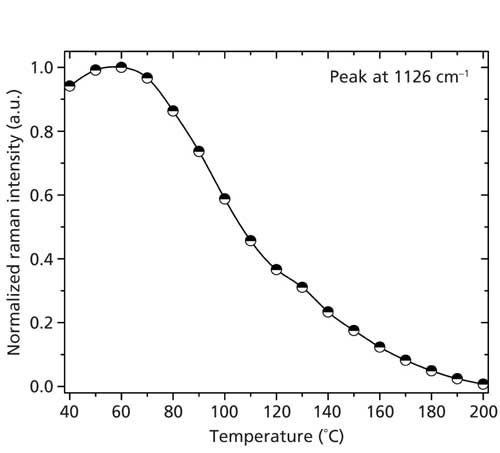
Figure 3: Normalized intensity (peak area) of the Raman peak at 1126 cm-1 as a function of temperature, as obtained from the full range Raman spectra. Note the intensity decrease with increasing temperature; above 170 °C, this intensity goes to zero.
An analysis of the Raman spectra obtained with the high-resolution setup, shown in Figure 4a, confirms that the Raman intensity decreases to zero just like in the case displayed in Figure 3. Figure 4b clearly shows the reversibility of the Brill transition during a heating and cooling cycle, since the Brill Raman bands emerge again when the sample cools below TB.
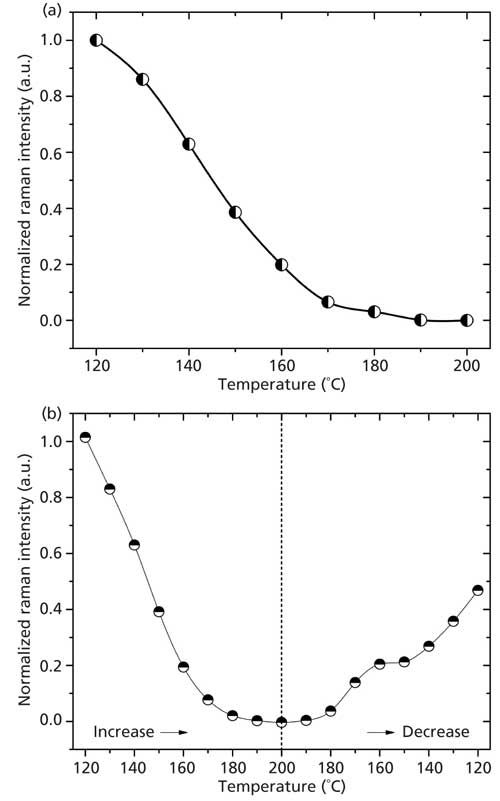
Figure 4: Normalized intensity (peak area) of the Raman peak at 1126 cm-1 as a function of temperature, as obtained from the “high resolution” Raman spectra. (a) For increasing temperatures from 120 °C to 200 °C it is possible to see that intensity decreases with increasing temperatures; above 170 °C this intensity converges to zero. (b) For a cycle with increasing and then decreasing temperatures between 120 °C and 200 °C it is possible to see that the Brill transition is a reversible process, since the band at 1126 cm-1 (Brill Raman band) emerges again when the temperature of the sample decreases below the Brill temperature.
Conclusions
We used Raman spectroscopy to verify the Brill transition and its reversibility in nylon 6,6, the transition happening in the temperature range between 160 °C and 180 °C (7,13). Raman spectra of nylon 6,6 were collected during a heating–cooling cycle in a temperature range between 120 °C and 200 °C, the Brill Raman bands at 1126 cm-1 and 1479 cm-1 being characteristic for the Brill transition. From their Raman intensity data plotted as function of temperature it is possible to conclude that the Brill transition occurs in a temperature range between 120 °C and 200 °C, the Brill Raman bands practically disappearing above 180 °C. We believe that the disappearance of these bands is associated with local melting of the methylene segments, as suggested by Vasanthan and colleagues (7), influencing the skeletal C-C vibration. The reversibility of the intensity data of the Brill Raman bands at 1126 cm-1 and 1479 cm-1 when cooling from temperatures above 180 °C demonstrate that Raman spectroscopy can be successfully used to verify the Brill transition.
References
- N.G. McCrum, B.E. Read, and G. Williams, Anelastic and Dielectric Effects in Polymeric Solids (Wiley, London, 1967).
- N.S. Murthy, S.A. Curran, S.M. Aharoni, and H. Minor, Macromolecules24, 3215–3220 (1991). http://dx.doi.org/10.1021/ma00011a027.
- N.S. Murthy, Z.-G. Wang, and B.S. Hsiao, Macromolecules32, 5594–5599 (1999). http://dx.doi.org/10.1021/ma990475e.
- R.J. Brill, Journal für Praktische Chemie161, 49–64 (1942). http://dx.doi.org/10.1002/prac.19421610104.
- H.W.J. Starkweather, Nylon Plastics Handbook (Hanser, Munich, Germany, 1995), p. 139.
- H.W. Starkweather Jr. and G.A. Jones, Polym. Sci. Polym. Phys.19, 467–477 (1981). http://dx.doi.org/10.1002/pol.1981.180190307.
- N. Vasanthan, N.S. Murthy, and R.G. Bray, Macromolecules31, 8433–8435 (1998). http://dx.doi.org/10.1021/ma980935o.
- C. Ramesh, A. Keller, and S.J.E.A. Eltink, Polymer35, 5293–5299 (1994). http://dx.doi.org/10.1016/0032-3861(94)90482-0.
- H.-J. Biangardi, J. Macromol. Sci., Part B: Phys.29, 139–153 (1990). http://dx.doi.org/10.1080/00222349008245770.
- J. Hirschinger, H. Miura, K.H. Gardner, and A.D. English, Macromolecules23, 2153–2169 (1990). http://dx.doi.org/10.1021/ma00210a009.
- S.J. Cooper, M. Coogan, N. Everall, and I. Priestnall, Polymer42, 10119–10132 (2001). http://dx.doi.org/10.1016/S0032-3861(01)00566-3.
- N. Sharma, B.K. Tripathi, A.K. Shrivastava, and R.S. Chauhan, IJEMS5, 128–139 (2014).
- J. Hirschinger, H. Miura, K.H. Gardner, and A.D. English, Macromolecules23, 2153–1269 (1990). http://dx.doi.org/10.1021/ma00210a009.
D. Bertoldo Menezes is with the Federal Institute of Triângulo Mineiro in Minas Gerias, Brazil and the Department of Chemistry and Physics of Materials at the University of Salzburg in Salzburg, Austria. A. Reyer and M. Musso are with the Department of Chemistry and Physics of Materials at the University of Salzburg. Direct correspondence to: durval@iftm.edu.br

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.