Chemical Warfare Agents and Use of Thermal Desorption–GC–MS to Achieve Improved Trace-Level Detection
Special Issues
This article discusses the analysis of a wide range of CWAs at current exposure limits and describes a number of recent beneficial developments in TD and associated analytical technologies for the identification and quantification of CWAs at these levels.
The Chemical Weapons Convention (CWC) (1993) outlawed chemical warfare agent (CWA) use and specified that destruction of agent stockpiles should advance within set deadlines. Ongoing CWA-related research projects (for example, civil defense, agent destruction, and protection) require monitoring of airborne CWA concentrations at the lowest possible level and with accurate identification. The use of handheld devices in these situations is compromised by their lack of sensitivity and their tendency to give false positives. Thermal desorption (TD), combined with GC–MS, is the only technique offering sufficient sensitivity and specificity for identifying and measuring trace CWAs. This article discusses the analysis of a wide range of CWAs at current exposure limits and describes a number of recent beneficial developments in TD and associated analytical technologies for the identification and quantification of CWAs at these levels.
Chemical warfare is defined as "Warfare and associated military operations involving the employment of lethal and incapacitating munitions and agents, typically poisons, contaminants, and irritants." This definition only covers deliberate use of agents and excludes most of those used legally, such as herbicides, smoke, and flame. An exception to this is the use of riot-control agents during warfare, which is now forbidden (1,2).
Chemical warfare has been used since ancient times. The "modern" era of chemical weaponry began in World War I (WWI) with blister agents such as mustard gas and continued with the advances in synthetic organophosphate chemistry during the development of insecticides in the 1930s.
Since then, thousands of chemicals have been screened as weapons. The current list of chemical warfare agents (CWAs) is divided into five groups, based roughly on mode of action: nerve, blister, choking, blood, and lachrymatory.
Toxicity and Protection
Typically, CWAs are liquids, or vapors dispersed as aerosols or gas. They can enter the body via the respiratory system or through skin contact. Nerve agents can kill within minutes of inhalation and in under an hour after skin contact. VX (Figure 1) and the Russian equivalent, RVX, are among the most toxic compounds in existence. A 1-L volume of VX can theoretically kill one million people, and less than one tenth of the volume of an average swimming pool could wipe out the entire human population (3). Moreover, VX persists on material, equipment, and terrain, remaining deadly for up to three weeks. Table I shows the maximum exposure limits for particular agents and illustrates their extreme toxicity.
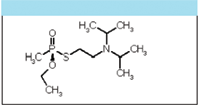
Figure 1: Chemical structure of VX.
It was quickly realized that the only real prospect for defense against chemical weapons was to ban their use. This prompted international action on a chemical disarmament regime under the 1925 Geneva Protocol. This protocol has limitations, however, including the inability to enforce policing and the fact that it does not forbid retaliation in the case of chemical attack. The Chemical Weapons Convention (CWC) addressed these shortfalls and has now been adopted by over 120 countries. The agreement involves not using or making (or encouraging others to make) chemical weapons. To prove this good intention, all parties agree to inspection of any site where agents might have been used. Another condition was the disposal of all large stockpiles by 2007. While this target was not met, international efforts to destroy agent stockpiles are proceeding at pace, with extensions until 2012 for substantial stockpiles and longer in other cases (4).

Table I: Maximum limits of human exposure to select CW agents
Russia and the U.S. possess more than 95% of the Cold War stockpiles of chemical arms. Albania, Libya, South Korea, and India have declared much smaller quantities, and Japan has started elimination of the CW stockpile abandoned in China after World War II.
Detection and Identification
Because CWAs are no longer in development, current research is focused on civil defense, personal protection, agent destruction, and antidotes. Many ongoing CWA-related research projects and processes require monitoring of airborne CWA concentrations at the lowest levels, often in highly contaminated environments such as subway stations. Accurate identification can also be critical — for example, in civil defense situations — so that remedial action and antidotes can be deployed rapidly to minimize casualties. Conventional handheld devices are not useful in these situations, as they are prone to false positives and signal the cue for evacuation at levels that are already lethal. Trace-level monitoring of CWAs in complex real-world environments requires speciated and sensitive chemical analysis.
Thermal desorption was introduced in the early 1980s as a front-end technology for gas chromatography (GC) and GC–mass spectrometry (MS). It involves the thermal desorption/extraction of organic vapors from sorbent tubes using heat and a flow of pure inert gas. After primary desorption, analytes are refocused before rapid secondary desorption and injection into the GC–MS analytical system as a concentrated "band" of vapor. Initial applications for TD-GC–MS included workplace and environmental air monitoring for conventional toxic chemicals. The technique was quickly adapted for agent analysis, both for personal exposure assessment (such as in monitoring personnel manning agent destruction facilities) and for checking airborne concentrations.
At first, however, TD systems were not compatible with the most demanding CWA, such as VX, nor did they offer sufficient sensitivity to allow detection at the lowest general population limit (GPL) levels. Traditional TD-GC–MS methods for VX, for example, involved derivatization to the "G-analog," using silver fluoride pads in front of the sampling line or sorbent tube. The derivatized compound is more volatile, which improves recovery, but sensitivity and repeatability are compromised because derivatization efficiency can be variable and is less than 100%.
In contrast to this approach, the latest chemically inert TD technology can be used for direct analysis of free-VX. Figure 2 shows the range of CWAs that can be analyzed simultaneously using one such system.
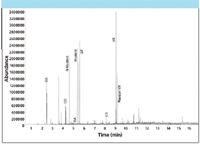
Figure 2: Analysis of mixed CWA standards (5 ng level) using TD-GCâMS.
On-line and off-line TD-based air monitoring protocols have now been developed for CWA analysis. On-line (near real-time) monitoring is performed at fixed installations such as storage bunkers and in mobile installations such as first-responder vehicles (Figure 3). Mobile laboratories can also be positioned near key public buildings for surveillance.

Figure 3: Mobile laboratory.
TD platforms for on-line monitoring typically comprise two reciprocally operated focusing traps. One samples airborne contaminants while the other is desorbed and analyzed, and vice versa (Figure 4). This "twin trapping" feature allows continuous near-real-time monitoring without data gaps.

Figure 4: Twin reciprocally operated focusing traps for continuous monitoring.
On-line monitoring is needed at military installations, destruction facilities, and key public buildings as first-line defense against a potential terrorist attack. Example data obtained on-line are shown in Figure 5.
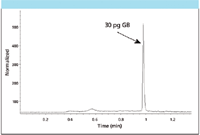
Figure 5: CWA (sarin) monitoring using online thermal desorption with GCâflame photometric detection.
Off-line monitoring is used for personal exposure assessment (for example, of military personnel guarding storage bunkers), confirming on-line data, and for research into protective equipment, decontamination procedures, and so forth. Off-line TD systems operate in two stages, with an initial larger sorbent trap/tube being used for maximizing air sampling flows and volumes and a secondary electrically cooled refocusing trap capable of fast desorption and injection of analytes into the GC–MS analyzer for optimum sensitivity. Figure 6 illustrates off-line detection of free-VX at typical GPL levels using an off-line TD-GC–MS system.
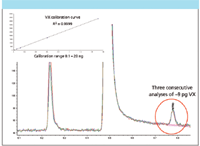
Figure 6: Off-line analysis of free VX from sorbent tubes. Inset shows linearity over a wide concentration range.
Figure 6 shows free-VX linearity over a wide concentration range (inset) combined with excellent repeatability at low picogram levels (see overlaid chromatograms). This illustrates the reproducibility and effectiveness of modern TD-GC–MS systems for this very challenging application.
Technological Developments for Enhanced TD-GC–MS Analysis of CWAs
Many of the most recent developments in TD and associated technologies complement the on-line and off-line monitoring protocols discussed above and offer significant benefits to trace detection and identification of CWAs. Relevant innovations include
- Electronic tagging of sorbent tubes and personal exposure monitors for improved traceability. The RFID technology shown in Figure 7, for example, can be automatically written to during field monitoring to minimize transcription errors and is automatically updated during laboratory analysis.

Figure 7: Electronic tags fitted to tubes as direct replacement for bar codes and manual etching and recording of tube identification numbers.
- Time-of-flight (TOF) mass spectrometers for GC, capable of providing full mass spectral information at traditional selected ion monitoring (SIM) detection limits. This is critical for the elimination of false positives at trace levels in complex sample matrices. The latest compact, benchtop systems offer exceptional mass stability for extended periods (Figure 8).
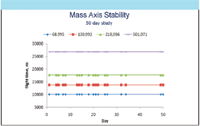
Figure 8: Mass stability over extended time periods of a bench-top compatible TOF-MS system for GC.
- A novel approach to GC–MS background compensation that responds dynamically as the analytical background changes. As described elsewhere (5), the new software can accurately distinguish between background and peak-related information even if the same mass ions are present in both. Its use dramatically improves signal to noise (sensitivity) and spectral purity (identification) for trace components (Figure 9) without compromising the original data file.
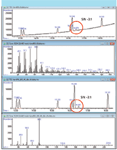
Figure 9: Illustration of spectral dynamic background compensation of thiophene. Raw data are shown in the upper panel, and reprocessed data is shown in the lower panel.
- New GC–MS "data mining" software is also under development for enhanced detection of trace target analytes in complex chromatograms. One such novel software approach combines dynamic baseline compensation (5) with spectral deconvolution and pattern recognition. While this approach is not yet available commercially, early results have been encouraging.
- Specialized equipment has also been developed for simplifying and extending the vapor sampling process. One example is a micro-chamber/thermal extractor (Figure 10) for testing material emissions and permeation. Multisample units like this interface to standard sorbent tubes and can be used to test the performance of safety equipment and the efficacy of decontamination procedures — for example, to investigate CWA residues on treated and untreated surfaces.
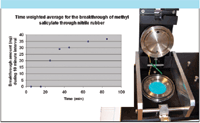
Figure 10: Example micro-chamber system in use for permeation testing. Inset shows breakthrough of a CWA simulant through a nitrile rubber membrane.
- Mechanically simple approaches to the automation of TD and associated sorbent tube sampling have also recently enabled the development of robust, high-throughput analytical systems and field sampling units.
The challenge for TD automation always has been to keep sampled and desorbed tubes sealed except during desorption and analysis but without the use of complex robotic procedures such as capping and uncapping, because these can compromise system reliability. The latest automated TD systems employ tube end caps incorporating a "diffusion locking" mechanism. This valve-free innovation prevents ingress of contaminants or outgassing of analytes via diffusion when the tubes are in standby but allows gas to pass when pressure or a vacuum is applied. Figure 11 shows the diffusion locking end caps deployed on sorbent tubes installed in an automated tube sampling device.

Figure 11: Field portable automated sampling device.
- Quantitative sample re-collection for repeat analysis has also been implemented on some of the latest commercial TD systems and provides a powerful and convenient approach to validating analyte recovery through the thermal desorber (Figure 12).
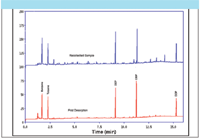
Figure 12: Re-collection to illustrate reproducibility in analyte recovery through a TD system.
Depending on the method of implementation, some modern thermal desorbers allow all or part of any sample split to be quantitatively re-collected on a conditioned sorbent tube after passing through the TD system. A sequence of repeat analyses can thus be performed on a single sample, allowing users to readily monitor analyte recovery relative to split ratio and to other components in the mix.
Summary
The innovations in TD-GC–MS technology for CWA monitoring described in this article have all been implemented over the last decade and have made a significant contribution to improving the performance and reliability of monitoring technology in this challenging application area.
On-line and off-line monitoring systems are now able to offer much improved data quality at ultratrace levels, which provides military and civilian authorities alike with the tools required to monitor demilitarization processes effectively and to keep us all that little bit safer.
Terry Murphy, Gareth Roberts, and Gavin Davies are with Markes International, Ltd., Llantrisant, UK.
References
(1) T. Taylor, International and Comparative Law Quarterly 42, 912–919 (1993).
(2) http://www.tinyurl.com/dn2479
(3) N. Munro, Environ. Health Perspect. 102(1), 18–37 (1994).
(4) R. Trapp, Ann. N.Y. Acad. Sci. 1076, 527–539 (2006).
(5) D. Rosser, G. Roberts, and L. Woolfenden, The Column, 20–24 (2008).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.