EDXRF Analysis of Sulfur and Trace Element Content in FAMEs
Special Issues
The EDXRF method described here is a quick and economical procedure to check samples for their oxygen content to ensure that the material used for diesel fuel blending is really FAME-based and not mineral-oil based.
Fatty acid methyl esters (FAMEs) are usually derived from vegetable oil and are typically blended with regular diesel fuel in the 5–20% range. An elemental analysis of pure FAMEs, used vegetable oil, or the reprocessed vegetable oil as well as biofuel blends is required to show compliance with the relevant specifications. Several test methods are available that describe the use of energy-dispersive X-ray fluorescence (EDXRF) for the analysis of sulfur in fuel, such as International Organization for Standardization (ISO) 13032 and ASTM International D7220. The current test methods, using EDXRF, are limited to fuels with a discrete maximum amount of FAME or oxygen. This article describes a quick and economical procedure to check samples for their oxygen content to ensure that the material used for diesel fuel blending is really FAME-based and not mineral-oil based. The oxygen content determined by this approach is used to apply the required matrix correction without a priori knowledge (which is typically required using other approaches), improving sulfur content determination accuracy. In addition to the analysis of the sulfur content, this article describes EDXRF as a technique to monitor other elements, including P, Cl, Na, K, Mg, and Ca, among others, that can have detrimental effects on combustion engines.
These days, renewable fuels and other biofuels play a growing role in the offer of automotive fuels. Currently, fatty acid methyl esters (FAMEs), mostly derived from vegetable oil, are typically blended with regular diesel fuel in the 5–10% or 20% range. An alternative for the fairly expensive fresh vegetable oil is recycled vegetable oil derived from the food industry. An elemental analysis of the pure FAMEs, the used vegetable oil, the reprocessed vegetable oil, and the biofuel blends is required to show compliance with actual specifications.
Typically, this type of analysis is done using inductively coupled plasma–optical emission spectrometry (ICP-OES) using the following test methods:
· EN14538-2006 (1) describes the analysis of Ca, K, Mg, and Na in FAME, samples require a 1:1 dilution (50%) in kerosene (to reduce matrix effects) and no internal standard is used.
· EN16294-2012 (2) describes the analysis of P in FAME, samples require a 1:3 dilution (25%) in kerosene and the use of an internal standard is mandatory.
Sulfur is not part of these methods and is determined using different technologies. The analysis requires some sample preparation and investment in purchase and operating cost of the analyzer.
This report describes a quick and economic alternative to quantify the sulfur content in FAMEs using energy-dispersive X-ray fluorescence (EDXRF).
Besides the analysis of the sulfur content, EDXRF equipment (depending on the analyzer performance), is capable of monitoring additional elements such as P, Cl, Na, K, Mg, Ca, and many more that can be harmful to car engines.
Recycled vegetable oils can be added into diesel fuel and used as secondary burner fuel. An elemental analysis to show the absence of toxic elements is part of the quality control. This can also be done with the XRF equipment.
In addition, it would be of interest to at least check the samples for their oxygen content to make sure that the material used for blending in the diesel fuel is really based on FAMEs and not based on mineral oil.
Test Methods
Several test methods are available that describe the use of EDXRF for the analysis of sulfur in fuel. The International Organization for Standardization (ISO) method 13032 (3) and ASTM International method D7220 (4) are two of them.
The currently available test methods using EDXRF are limited to fuels with a discrete maximum amount of FAME or oxygen. In the example of ISO 13032, the maximum content of FAME is 10% or 3.7% of oxygen. The reason is that the oxygen has a matrix effect on the sulfur signal measured by XRF. To be able to use XRF for this type of analysis, various approaches are possible:
· Correction using defined correction values depending on the known oxygen content, which requires a priori knowledge of the oxygen content.
· Correction using fundamental parameters based on the matrix, which has to be predefined, and which also requires a priori knowledge of the oxygen content.
· Correction using backscatter information from the sample. This approach does not require any a priori knowledge of the sample matrix, but the backscatter correction must be based on a signal, which should be close to the analyte’s X-ray fluorescence energy because otherwise additional effects occur.
· Additional references to test methods are also available (5–7).
For other elements of interest, the same effect is visible. The influence on the results varies depending on the element.
Instrumentation
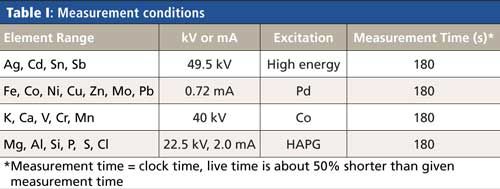
Our procedure used a Spectro Xepos analyzer in which samples were excited by a forced-air-cooled 50 W end-window X-ray tube combined with a doubly curved HAPG crystal for monochromatization and polarization of the primary tube spectrum. In addition, direct excitation using Pd-K and Co-K radiation can be used to optimize the excitation conditions for groups of elements. A high-resolution silicon drift detector (SDD) was used to collect fluorescence radiation from the sample. The highly stable spectral resolution of the SDD was <130 eV for Mn Kα.
The measurement parameters are given in Table I.
Sample Preparation
The sample preparation for the analysis is straightforward: 4 g of sample material is poured into a disposable XRF sample cup with an outer diameter of 32 mm. The analytical side of the sample cup is closed with a 4-µm-thick polypropylene film. For additional safety, the sample cups are placed into a sample holder, which is closed with a second polypropylene film of the same thickness.
Applications
As explained above, biodiesel blends according to specification EN 590 typically can be analyzed with a calibration prepared for diesel fuel. Using the same calibration for the analysis of a biofuel sample with 12.5% oxygen would lead to a significant bias in the analysis result as can be seen in Figure 1.
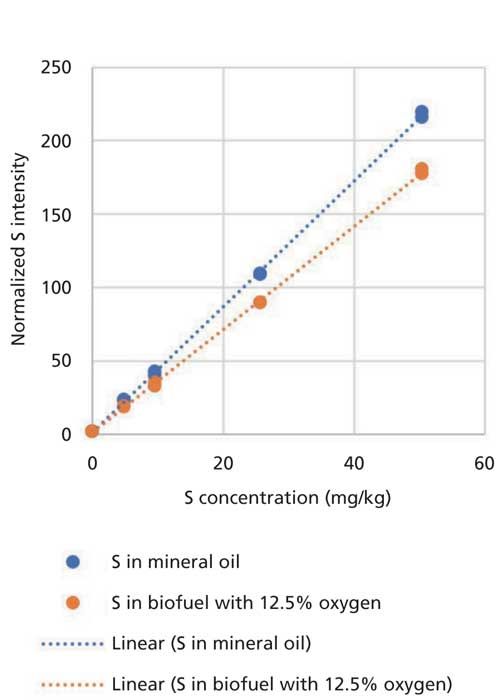
Figure 1: Calibration for S in mineral oil and biofuel with 12.5% oxygen.
In many cases the oxygen content of the sample being analyzed is not known, and therefore the best option for an appropriate matrix correction is to determine the oxygen content of the sample. Using XRF, the direct determination of the oxygen content in fuel is not possible because the fluorescence energy of oxygen is very low and is not detectable.
The second approach is to use the backscatter information of the sample to indirectly determine the content of oxygen. The so-called Compton scatter signal will decrease with increasing O-content. To be able to achieve this accurately, the energy of the Compton scatter signal should be at relatively low energy because otherwise volume effects will affect the determination.
Since the EDXRF system used in our procedure is equipped with an X-ray tube with a thick binary Pd-Co alloy anode, in principle, there are two options to use backscatter information: one from the excitation using the characteristic radiation of Pd and the second using the characteristic radiation of Co.
Because of the reasons listed above, the cobalt excitation of the EDXRF system can be used for this determination with good accuracy. Figure 2 shows the calibration of the measurements of samples with known oxygen concentration.
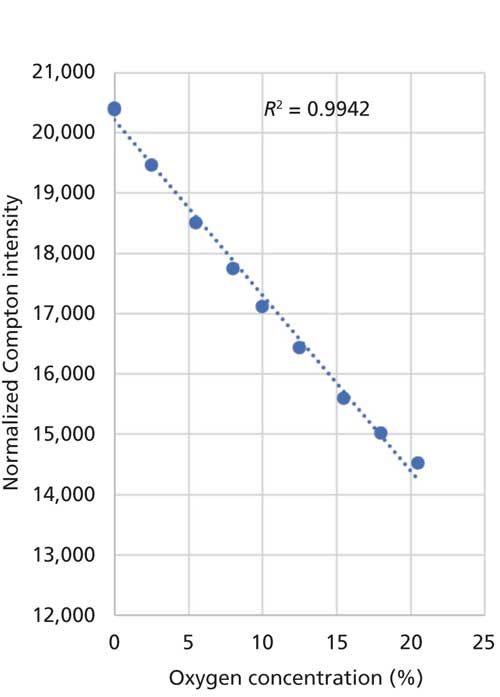
Figure 2: Calibration for O in fuels.
The figure demonstrates the sensitivity and accuracy of this indirect determination of the oxygen content. Based on this determination, the differentiation between materials based on FAMEs or on mineral oil is easily possible.
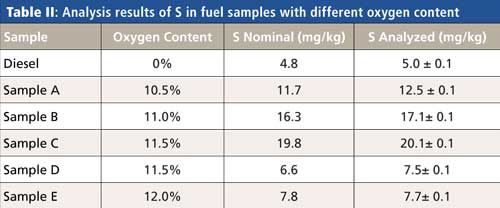
For the accurate determination of the trace element content, the oxygen content determined with this procedure is used within a fundamental parameters program. Table II shows some examples.
As previously listed, other trace elements can be determined simultaneously using EDXRF. Table III gives detection limits for additional trace elements.
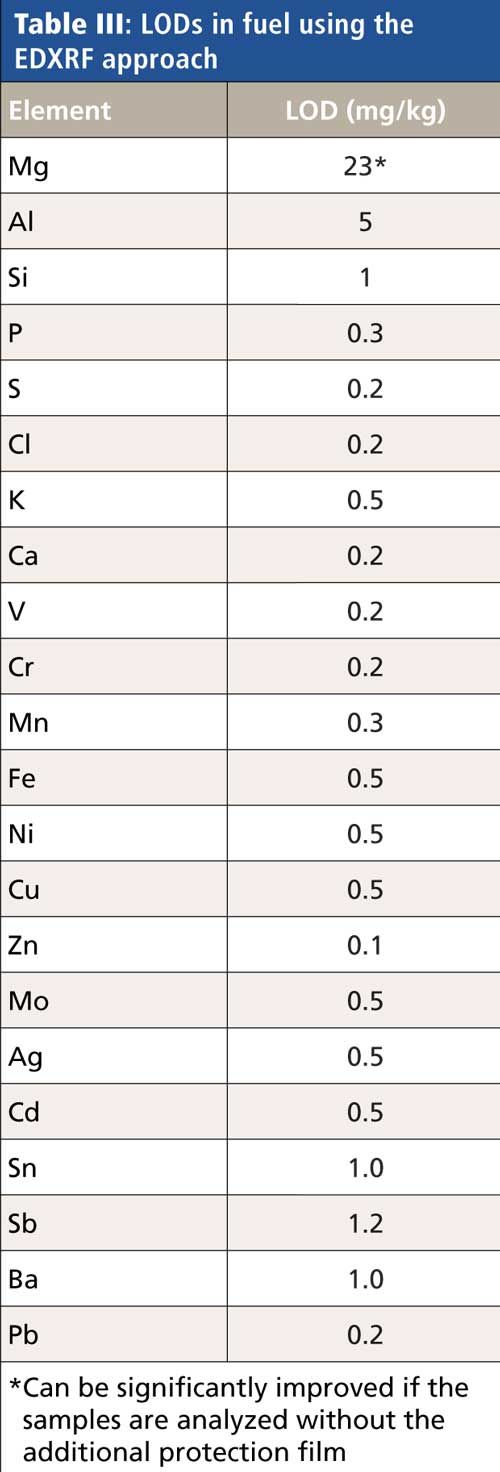
Although there is currently no specified test method using XRF for the determination of these elements in biofuels, the listed limits of detection (LODs) clearly demonstrate that at least a screening for the content of these elements is possible using the EDXRF, even at low levels.
Summary
EDXRF clearly can be used for the analysis of S and other trace elements in biofuel. The determination of the oxygen content helps to compensate for matrix effects efficiently, and a priori knowledge of the oxygen content is not required.
References
- EN14538-2006: Fat and oil derivatives. Fatty acid methyl ester (FAME). Determination of Ca, K, Mg and Na content by optical emission spectral analysis with inductively coupled plasma (ICP-OES).
- EN16294-2012: Petroleum products and fat and oil derivatives. Determination of phosphorus content in fatty acid methyl esters (FAME). Optical emission spectral analysis with inductively coupled plasma (ICP-OES).
- International Organization for Standardization, ISO 13032, “Petroleum products – Determination of low concentration of sulfur in automotive fuels – Energy-dispersive X-ray fluorescence spectrometric method” (ISO, Geneva, Switzerland, 2012).
- ASTM International, D7220, “Standard Test Method for Sulfur in Automotive, Heating, and Jet Fuels by Monochromatic Energy Dispersive X-Ray Fluorescence Spectrometry” (ASTM International, West Conshohocken, Pennsylvania, 2017).
- European Committee for Standardization (CEN, French: Comité Européen de Normalisation) http://www.cen.eu/cenorm/.
- http://www.astm.org or contact ASTM Customer Service at service@astm.org.
Dirk Wissmann is the senior product manager and product manager for XRF at Spectro Analytical Instruments GmbH in Kleve, Germany. Direct correspondence to: dirk.wissmann@ametek.com

AI Shakes Up Spectroscopy as New Tools Reveal the Secret Life of Molecules
April 14th 2025A leading-edge review led by researchers at Oak Ridge National Laboratory and MIT explores how artificial intelligence is revolutionizing the study of molecular vibrations and phonon dynamics. From infrared and Raman spectroscopy to neutron and X-ray scattering, AI is transforming how scientists interpret vibrational spectra and predict material behaviors.
Advancing Corrosion Resistance in Additively Manufactured Titanium Alloys Through Heat Treatment
April 7th 2025Researchers have demonstrated that heat treatment significantly enhances the corrosion resistance of additively manufactured TC4 titanium alloy by transforming its microstructure, offering valuable insights for aerospace applications.