External Reflection Analysis of Surface Coatings
Special Issues
External reflection using IR spectroscopy is effective for examining paintings, polymer-coated metals like soda can coatings, and even bulk polymers. Shiny, metallic surfaces provide a strong specular reflection spectrum that matches standard absorbance spectra very well. Less-reflective surfaces, like dark plastics, may require additional software processing.
The study of coatings on surfaces affects materials as diverse as soda cans and masterpieces of art. Fourier transform infrared (FT-IR) spectroscopy is ideal for this purpose, because many of those coatings, such as oil-based paints and polymeric films, are organic in origin, and FT-IR is inherently nondestructive. Additionally, the analysis can be completed without making contact with the surface, thus protecting a masterpiece painting or preserving the integrity of the films. We examine here a variety of materials, including polymeric coatings on metal and paint on canvas, using external reflectance. Although the technique was specifically designed for art conservation, we will show it to have utility in a variety of similar applications.
The need for noncontact analysis of surfaces extends over a wide range of applications. For instance, coatings such as paints or polymers are heavily used, performing cosmetic, artistic, or protective functions. Many of these materials have significant organic content, such as the polymeric coatings in soda cans or the oil-based materials used by painters. The analysis of these coatings provides insights into their integrity, robustness, or provenance, the latter being critical in cases of art preservation and fraud detection (1). Being able to nondestructively determine composition quickly and economically provides useful information to quality assurance (QA) and quality control (QC) laboratories, art conservators, and production facilities.
Fourier transform infrared (FT-IR) spectroscopy is an excellent investigative tool for this purpose. First, the sensitivity to organics is very high, especially with polymeric materials. In addition, FT-IR has low energy and low power, which make the probe itself nondestructive. The key is sample presentation. Many methods require sample chips to be removed for contact with an attenuated total reflectance (ATR) crystal. This process may damage a masterpiece painting or scratch a metal surface, exposing it to corrosive environments.
FT-IR reflectance has been successfully used in this setting for some time. For instance, a substantial amount of work was done using FT-IR to study the large frescoes seen in Pompeii (2). However, this required an FT-IR spectrometer or FT-IR microscope to be positioned near the fresco, or alternately that a piece of the fresco be supplied in a laboratory setting. We show here results obtained using a portable, noncontact spectrometer and accessory combination that grew out of a collaboration involving the art conservation community, with their specific and strict requirements. We start with the analysis of a painting, but we will then demonstrate the general utility of the external reflectance method.
Experimental
The data were collected using a ConservatIR FT-IR external reflection accessory (Thermo Scientific) in the sample compartment of a Nicolet iS5 (Thermo Scientific) FT-IR spectrometer. Since the system can be operated via battery, the entire system is portable and can be used in field locations such as archaeological sites or aircraft hangers. The accessory has an adjustable angle head that enables the analysis of ceilings, wall-mounted paintings, or engine surfaces.
The infrared beam is directed out of the sampling head to a focus about 1 cm away from the sampling head, with a spot size about 1.25 mm in diameter. In the work done here, both diffuse and specular reflectance data were collected. Visual images of the surface being analyzed were also collected using the built-in camera, providing the link between the target and the data.
A few of the spectra were processed using an atmospheric correction and the Kramers-Kronig (K-K)correction, as noted in the text. Backgrounds were collected from either clean surfaces (for the metallic materials) or a roughened white ceramic surface for the diffuse reflectance.
Results and Discussion
The requirements for these measurements were driven by a collaboration with art conservators. A typical question would be whether the formulation of the paints on a canvas are consistent with those in use by an artist or are indicative of a later date. In Figure 1, spectra collected from an oil painting at a few locations over the surface are shown. Oil paintings are not highly reflective in the infrared range, so the amount of light reaching the detector is low. Further, many of the bands appear with a "first derivative" shape because of the strong reststrahlen effect (3); note especially the ester band at 1750 cm-1. The refractive index changes sharply near a strong absorption band such as that of the ester, which prevents the IR light from being able to propagate through the heavy oil paints. The resulting "first derivative" bands can be corrected using the K-K correction (Figure 2). Note the sharp change in refractive index (second plot) around the strong peaks. The final spectrum now appears normal and the signal-to-noise ratio is quite good.
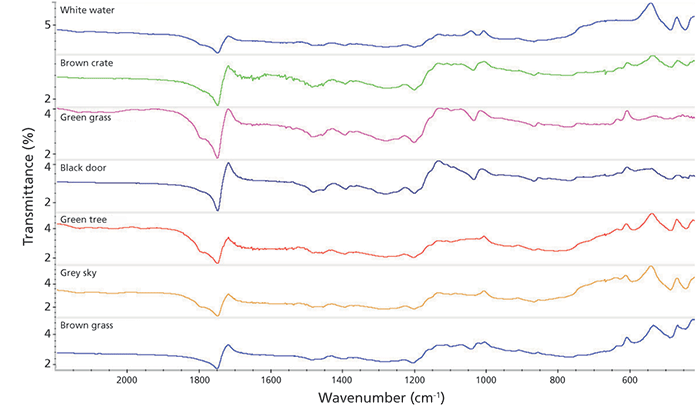
Figure 1: Spectra acquired from external reflection off a painting. The signature indicated a date in the early 1900s while the spectra indicate a later date.
The system was not purged for this application. The time between the background data collection and the sample collection was several minutes, and we did not apply atmospheric correction for this data set. Even so, the presence of the atmospheric interferences is weak. This lack of interference shows the system is stable, an important consideration when implementing external reflection through the large spectral sampling opening.
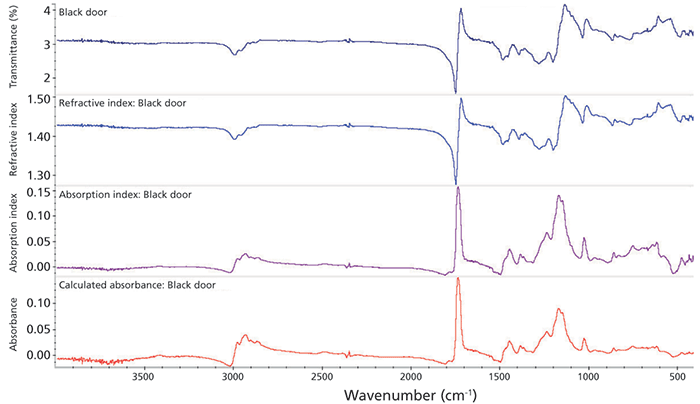
Figure 2: Kramers-Kronig correction applied to spectrum 16 (black door) from the painting. Top: Raw spectrum. Second: Refactive index. Note steep change around the ester region (1750 cm-1), which is what causes the reststrahlen bands. Third: Absorption index. Bottom: Calculated "transmission equivalent" spectrum. Search result against this spectrum yields close match to an acrylic resin used in printing and packaging.
The painter indicated by the signature on the painting worked in the early 1900s. Searching spectrum 16 (black door) against a set of commercial spectral libraries (industrial coatings, paints, inks) yields a strong match (>90) to a latex acrylic emulsion. This information was sufficient to rule out this painting being an original, because those materials did not become available until at least the 1930s. The remaining spectra show similar provenance-far too modern.
Next, we examine a more general application of coatings on metals that provide aesthetic and protective qualities. A good example involving both characteristics is a soda can. The inner wall requires corrosion resistance because of the acidity of the beverage, and the outside of the can provides a surface for colorful advertising and information. Figure 3 shows spectra collected from both the inside and outside surfaces of a soda can. These spectra were collected in specular reflection mode, and the signals are very intense in both cases with a very high signal to noise. Atmospheric interferences again are extremely minimal here, so no further processing is required.
The bottom spectrum in Figure 3 comes from the outside of the can, where the printing is present. Searching this spectrum against commercial libraries reveals a close match to an isophthalic polyester coating (note the large, intense band near 1750 cm-1). This type of resin has a very high resistance to moisture, which clearly is an advantage in this specific application.
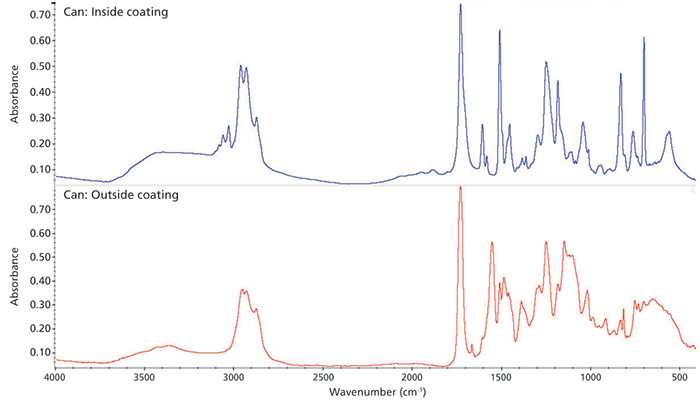
Figure 3: Spectra from the inside (top spectrum) and outside (bottom spectrum) of a soda can, acquired using specular reflection.
The spectrum of the coating from the inside (top spectrum in Figure 3) is quite different. Again, the specular reflection spectral peaks are intense and the spectrum is very clean. The best search result is an epoxy resin with tung and castor oils. This coating would be in keeping with the movement of manufacturers toward more biofriendly materials (4).
External reflection provides an easy-to-implement probe for industrial processes, such as examination of materials flowing into a recycling facility. In a very simple case, Figure 4 shows the spectrum from a piece of white packing foam. This spectrum is immediately identifiable as that of polystyrene.
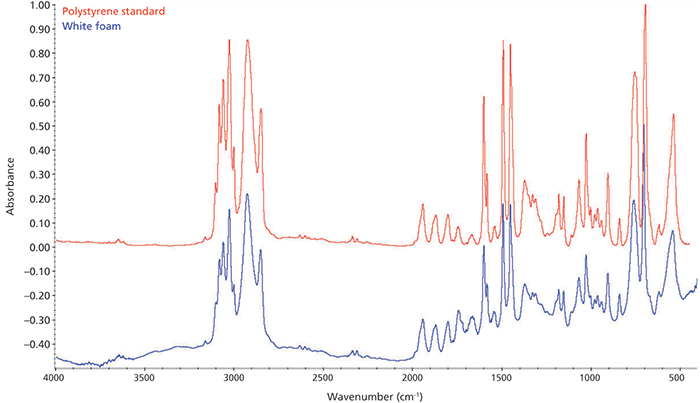
Figure 4: Spectrum of a white packing foam. The sample was handheld at the collection port of the accessory.
In contrast, external reflectance spectra taken from a dark plastic material exhibit reststrahlen bands as seen previously. The spectrum at the top of Figure 5 has undergone atmospheric correction to remove water and carbon dioxide bands. After K-K correction, the final absorbance spectrum is shown at the bottom. The final spectrum matches that of acrylonitrile-butadiene-styrene (ABS) in a spectral search. The telling peak in this analysis is the tiny, but clearly visible, band at 2234 cm-1 that results from the nitrile group (C≡N), a unique absorption band that could be obscured by the carbon dioxide or noise. The observable presence of this band illustrates the responsiveness of external reflection. This quality was exploited recently in an industrial implementation of external reflectance in an automotive production plant QA/QC setting, where the need for a metal surface to be scrupulously clean before sealing is tested by external reflection off the metallic surface. Tiny traces of oils or metal treatment were detectable, leading to a significant improvement in throughput and reliability of the engine parts.
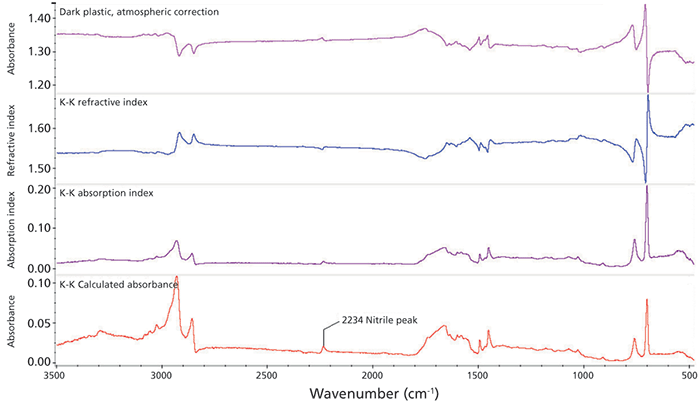
Figure 5: Spectrum from a dark plastic piece. Atmospheric correction was applied first, then the Kramer-Kronig correction. The small nitrile band at 2234 cm-1 is clearly seen, helping prove the sample is ABS plastic.
Conclusion
External reflection is shown to be effective for examining paintings, polymer-coated metals, and even bulk polymers. The responsiveness depends upon the surface. Shiny, metallic surfaces provide a strong specular reflection spectrum that matches standard absorbance spectra very well. Less-reflective surfaces, like dark plastics, may require additional software processing, such as atmospheric correction (which would likely need to be done in a harsh manufacturing environment) and the K-K transform. The advent of efficient and flexible external reflection analysis solutions on portable platforms will simplify data collection and add to their utility.
References
(1) J.G. Grasselli, Anal. Chem. 55(8), 874a–880a (1983)
(2) R. Piovesan, R. Siddall, C. Mazzoli, and L. Nodan, J. Arch. Science 38(10), 2633–2643 (2011)
(3) P.R. Griffiths, J.A. de Haseth, and J.D. Winefordner, Fourier Transform Infrared Spectroscopy, 2nd Ed. (Wiley, Hoboken, New Jersey, 2007).
(4) G.S. Sudha, H. Kalita, S. Mohanty, and S.K. Nayak, Int. J. Polym. Anal. Charact. 22(6), 519–525 (2017).
Michael Bradley is with Thermo Fisher Scientific in Madison, Wisconsin. Direct correspondence to: mike.bradley@thermofisher.com

Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.
The Rising Role of Near-Infrared Spectroscopy in Biofuel Innovation
July 25th 2025A new bibliometric study published in Infrared Physics & Technology highlights the growing global impact of near-infrared (NIR) spectroscopy in biofuel research, revealing key trends, contributors, and future directions for advancing sustainable energy solutions.
Best of the Week: The Emerging Leader in Molecular Spectroscopy, Big Pharma’s Manufacturing Shift
July 25th 2025Top articles published this week include a feature article about big pharma’s investments in U.S.-based manufacturing, an article about the 2025 Emerging Leader in Molecular Spectroscopy Lingyan Shi, and some news items detailing the winners of the Coblentz Society’s student awards.