Handheld FT-IR Spectroscopy for the Triage of Micro- and Meso-Sized Plastics in the Marine Environment Incorporating an Accelerated Weathering Study and an Aging Estimation
Spectroscopy
Four of the most prevalent commodity plastics were analyzed on-site using handheld FT-IR spectroscopy to determine changes correlated with accelerated weathering in the marine environment.
Debris in the marine environment can be either natural, such as floating vegetation or volcanic ash deposits, or man-made. The man-made sources cover the whole gamut of material types, such as sewage, glass, mineral, fabric, and, of increasing concern, plastic or polymeric materials. Virtually all plastics absorb infrared (IR) light in a highly selective manner, making their infrared spectra a useful qualitative diagnostic. The triage of the visible micro- (~1 mm to 5 mm), meso-, macro- or mega-sized plastic particles with handheld Fourier Transform IR (FT-IR) enables rapid determination of the material on-site, and reduces time wasted on non-polymers on-site or at site. Four of the most prevalent neustonic plastic types, and their FT-IR spectral changes correlated with accelerated weathering, were successfully examined chronologically, detailing significant differences in aging profiles and chemical changes. Subsequently, a small spectroscopically identifiable degraded piece of plastic found in Greenland was correlated to the appropriate aging profile. Finally, a targeted methodology for quantification of submillimeter microplastic in dried estuarine sediment was evaluated to ascertain the potential limit of detection.
Within a few decades of mass production of plastics in the 1940s, marine pollution was first reported in the early 1970s, but was largely inconspicuous. In the intervening years to the present, plastic pollution in the marine and coastal environment has received a tremendous amount of media, public, and scientific attention involving multiple scientific disciplines. To date, there are several excellent reviews covering the physical impact (1), plastic generation and potential impacts (2), identification and quantification (3), sources, distribution, and fate (4), and mitigation measures (5), as well as reviews of multiple studies (3,6). In all reviews, the actual particles that were further graded by a diagnostic qualitative instrumental technique were limited to a quotient of the full number of suspected particles. The lack of standard practice related to the quantification of plastics in the environment is a subject that is well covered in the aforementioned reviews.
In the analysis of plastics in the marine environment, primary sorting after sieving begins with visual sorting and separation and classified by their physical type (fragment, film, foam, pellet, and fiber) and their geometric aspects (including chips, spherical, round, and cylindrical). When used, secondary qualification via instrumental identification of the micro- and meso-sized plastics has involved a wide range of differing instrumentation with Fourier-transform infrared (FT-IR) spectroscopy being by far the most popular technique (3,6). When secondary confirmation by instrumental means is not used, the categorization is purely visual and physical.
The four most commonly found polymers in a study of 68 white papers (3) were found to be high density polyethylene (HDPE), low density polyethylene (LDPE), polypropylene (PP), and polystyrene (PS). Consequently, these polymers were chosen for an in-depth aging study to ascertain the aging profile, using a handheld FT-IR instrument to enable triage of samples in situ. In depth aging studies of HDPE (7), LDPE (8), and LLDPE (7) grades of polyethylene, polypropylene (9,10), and polystyrene (11) have been conducted. These studies concentrate on either physical or chemical changes induced by ultraviolet (UV) radiation and other possible degradation pathways (12,13). UV radiation and the availability of oxygen are the most important factors for initiating and propagating polymer degradation (12,13).
The occurrence of microplastics in the environment is far-reaching, covering oceans (14), rivers (15,16), bays (17), estuaries (18), lagoons (19), fauna and marine organisms (1,4,6,20), coastlines (21), and even activated sludges used in wastewater treatment plants (22), as well as a variety of freshwater systems (23). The prevalence of anthropogenic plastic is an increasing environmental concern with the potential to affect human food sources from both salt and fresh water environments. Moreover, increasing levels of degradation of specific polymers can lead to increasingly poor FT-IR identification.
In this proof of concept study, the main aim was to assess the benefits and extent of UV-initiated aging for each polymer, and the use or prevalence of these selected polymer types; to equivalently estimate the age of floating micro- and meso-sized plastics in the sea, rivers, beaches, sediments, and other marine environments in situ. Given that these polymers are highly likely to be prevalent in any significant localized concentration (such as oceanic gyres and other deposition sites), useful information can be gleaned from their individual aged states and their population variance. Consideration of the cross-correlated (all four types of polymer) quantification of the residence time of HDPE, LDPE, PP, and PS will collectively act as interpopulation verification.
Results and Discussion
Handheld FT-IR–ATR Aging Study Analysis
FT-IR is a well-documented technique for the identification of polymers. Because polymers degrade from the outside in, attenuated total reflectance (ATR) is particularly suited to the surface chemical changes. These environmentally induced chemical changes precede physical changes, and could be used as markers. The UV-induced changes for HDPE, LDPE, PP, and PS are shown in Figure 1. The spectra have been split into two aging segments, and stacked for clarity. The unaged HDPE and LDPE can be discriminated from the fine details in the methyl group symmetric deformation vibrational mode centered around 1369 cm-1, where the LDPE with the higher degree of branching contains a higher density of this functionality. For the accelerated aging 0 h to 442 h sector (Figure 1), both HDPE and LDPE show only slight to no changes, whereas the changes for PP are mild, and the changes for PS are especially evident in the carbonyl region at the latter analysis interval (442 h), where significant carboxylic acid and alcohol formation becomes evident. For the second sector (aging time 442 h to 1138 h), the HDPE only just begins to show changes toward the end of the aging experiment. LDPE changes are a little more severe, likely due to the higher degree of branching and, therefore, greater concentration of more reactive methyl groups. The even higher methyl concentration of PP results in discernible aging even earlier than LDPE around the 183 h mark. HDPE, LDPE, and PP share the same -C-C- backbone, and are fully saturated polymers; this affords them excellent UV resistance. Although PS also has the -C-C- backbone it also has an aromatic ring on every other carbon atom, causing PS to have the least UV resistance of the common neustonic polymers studied. Changes are apparent from early on and continue to increase in severity, until, at 1138 h, the spectra are unrecognizable from the starting material; by this point the PS is in a very advanced state of oxidation.
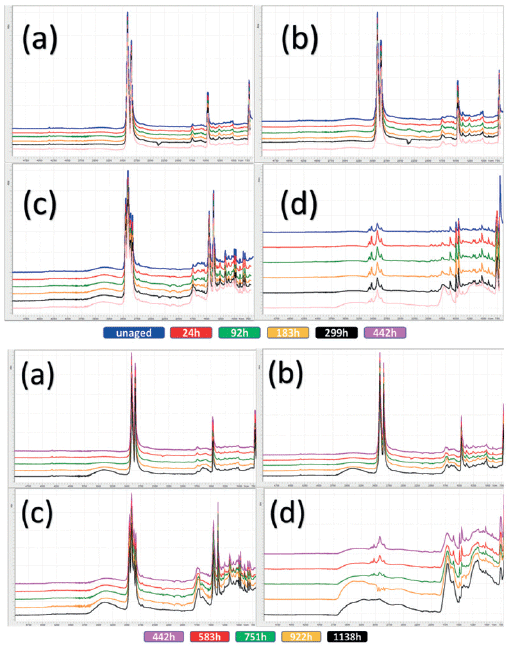
Figure 1: Each individual spectrum represents the normalized and averaged set per aging time and polymer type. Top set 0 to 442 hours, bottom set 442 to 1138 hours, for a) HDPE, b )LDPE, c) PP, and d) PS. Spectra are stacked and color coded for clarity and correspondence to color key is below each set.
The main spectral regions of change for the HDPE, LDPE, and PP samples are confined to hydroxlyation and carbonyl species formation, and these have been documented in several studies (7-10, 12,13) as only occurring toward the closing stages of the aging study. The PS aging, on the other hand, is much more complex, with a wider variety of chemical changes. Figure 2a shows the FT-IR peak area changes for the region from 3750–3,000 cm-1 for HDPE, LDPE, and PP, and a wider region of 3750–2250 cm-1 for PS, whereas Figure 2b shows the peak area changes for region 1850–1500 cm-1 for all four targeted polymers. The increase in area is concomitant with increasing chemical changes due to UV-induced oxidative and chemical degradation.
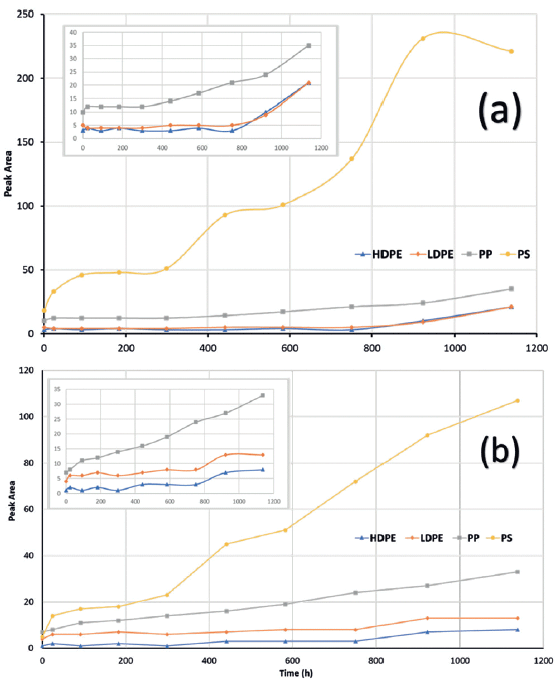
Figure 2: Peak area analysis of two zones: a) 3750-3,000 cm-1 for HDPE, LDPE, and PP; and 3750–2200 cm-1 for PS, and b) 1850–1500 cm-1 for HDPE, LDPE, PP, and PS. Increase in peak area signifies greater absorbance in that zone and greater degradation due to chemical changes. Inset graphs are zoomed in for the structurally similar HDPE, LDPE, and PP, excluding PS.
The spectra for both a green and a red piece of microplastic found on the northern coast of Greenland were measured by handheld FT-IR–ATR. Spectrally, the green piece was visually identified as being a degraded piece of PS, and gave a correct polymer identification library hit quality index (HQI) value of 0.84. This identification can be further confirmed by comparison of the degraded plastic with the set of accelerated aging spectra for PS, as shown in Figure 3. Unfortunately, the red piece of microplastic gave an incorrect match as wood with a HQI of 0.72. A speculative guess would be that the red microplastic may be an aged polyacetal.
To estimate the equivalent accelerated age of the green microplastic, the two zones used in Figure 2 peak area protocol were applied and then compared to the peak area value table for PS as shown in Table I, which includes the peak area values for all four neustonic polymers. The accelerated age is estimated to be equivalent to approximately 299 h. The fact that the marine environment aged sample has an increase in higher wavenumber changes is likely due to the salt water environment prior to beach deposition.
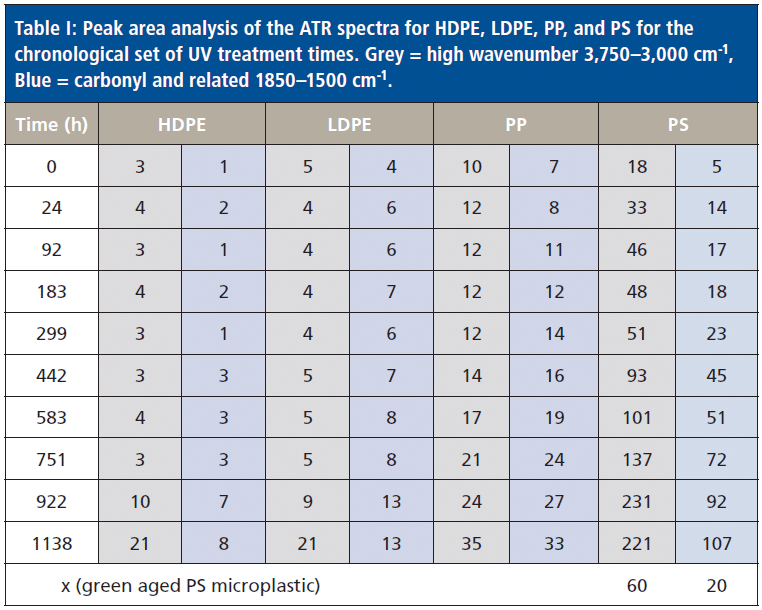
Table I: Peak area analysis of the ATR spectra for HDPE, LDPE, PP, and PS for the chronological set of UV treatment times. Grey = high wavenumber 3,750–3,000 cm-1, Blue = carbonyl and related 1850–1500 cm-1.
Both ATR and a dedicated diffuse interface were evaluated for a serial dilution factor of 2 for a dried estuarine sediment mixed with a formulated polymer blend; this was routed to achieve a particle size of less than 1 mm. The sizes were subsequently confirmed by optical microscopy. ATR is highly surface sensitive, and therefore had a very high limit of detection for a submillimeter microplastic sediment mixture. This was attributed to textural effects limiting adequate contact for the submillimeter microplastic where the acicular shape limited the area of contact. Parts a, b and c of Figure 4 show optical images of the pure sediment, polymeric formulation, and then a 50:50 mix by weight. The sediment is not only highly carbonated, but also fairly humic, whereas the polymer contains a small fraction of inorganic additives. This is, in effect, the worst-case scenario. The corresponding spectra are shown in Figure 4d.
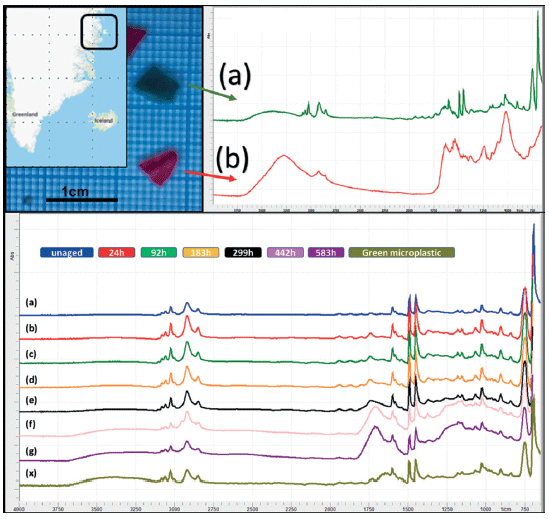
Figure 3: Top image shows environmentally aged microplastic from Greenland: a) ATR spectra of green PS microplastic sample, b) ATR spectra of red unidentifiable microplastic sample. Bottom image shows the set of accelerated UV-aged PS samples 0h–583 h (a–g), plus identifiable green microplastic "x".
Although ATR excels at polymer identification when the spectra and degradation state are available, it is not ideal for thoroughly mixed bulk mixtures. For such samples, the shallow depth of penetration for ATR is a hindrance rather than an advantage. Handheld diffuse reflectance, on the other hand, has nearly two orders of magnitude greater penetration depth and is much better suited to well mixed powder-like materials or ground or pulverized materials. The microplastic powder was serially diluted with a dilution factor of 2 to low ppm levels. Spectra were collected between each dilution and models were created as a proof of concept. The Beer-Lambert based limit of detection (LOD) was determined to be ~6.25% whereas the multivariate analysis (MVA) based partial least squares (PLS1) analysis was able to yield a LOD of around 0.4% for the microplastic using the same spectra, with a standard error of prediction (SEP) of 0.09%. Figure 5 shows the actual vs. predicted plot for the PLS1 model utilizing multiplicative scatter correction (MSC). Biological or environmental accumulation of polymeric submillimeter microplastics or suspected sites can be triaged with targeted analysis.
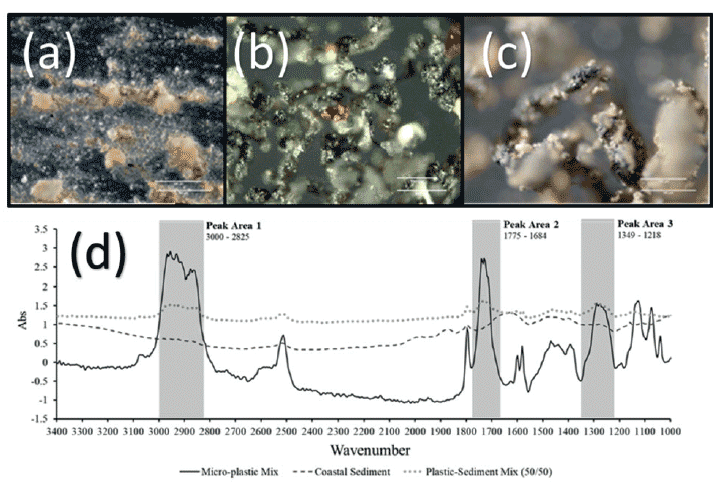
Figure 4: Optical micrographs of a) estuarine sediment, b) routed engineering plastic, and c) 50:50 mix. White scale bars are 150 µm and 180 µm, respectively, in each picture; d) represents handheld diffuse spectra of a, b, and c.
Conclusion
Degradation of the four most prevalent polymers accelerated aging profiles have been investigated and examined by handheld FT-IR–ATR. Where photo-initiated abiotic degradation dominates, these changes can be used for the semi-empirical quantification of the equivalent accelerated age of a real microplastic particle. This polystyrene microplastic example, collected on a beach in Greenland, a country with the lowest population density on the planet, was significantly aged. The accelerated UV aging directly confirms the spectroscopic chemical oxidative changes, and confirms the UV resistance order of HDPE>LDPE>PP>PS. The library hit quality index (HQI) results and the degradation profiles demonstrate the risk of over reliance on library searches alone, whereby polymers that age quickly are increasingly difficult to identify. A serially diluted ground polymer that was then mixed with estuarine sediment was found to have an LOD of about 0.4% weight for weight. The aging profiles can be further enhanced by the introduction of other degradation factors and other commodity polymers.
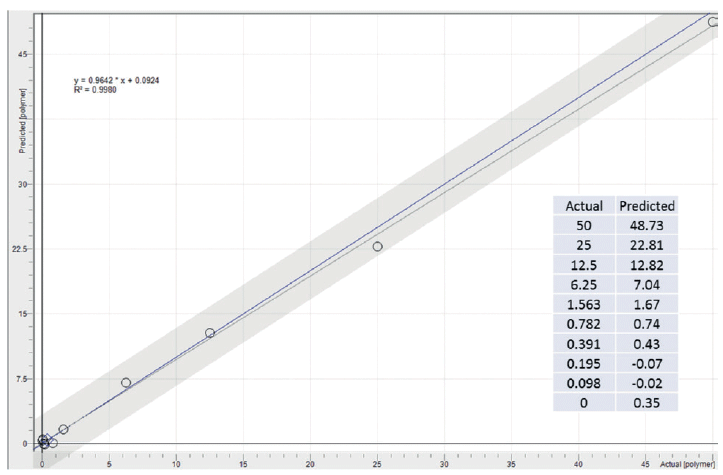
Figure 5: Partial least squares (PLS1) diffuse reflectance model for the percent submillimeter polymer content in a dried sediment,and their actual versus predicted values.
References
(1) S.L. Wright, R.C. Thompson, and T.S. Galloway, Environ. Pollut. 178, 483-492 (2013).
(2) A.L. Andrady, Mar. Pollut. Bull. 62, 1596-1606 (2011).
(3) V. Hidalgo-Ruz, L. Gutow, R.C. Thompson, and M. Thiel, Environ. Sci. Technol. 46, 3060-3075 (2012).
(4) K.L. Law, Annu. Rev. Mar. Sci., 9, 205-229 (2017).
(5) O.S. Ogunola, O.A. Onada, and A.E. Falaye, Environ. Sci. and Pollut. Res. 25(10), 9293-9310 (2018).
(6) S. Rezania, J. Park, M.F.M. Din, S.M. Taib, A. Talaiekhozani, K.K. Yadav, and H. Kamyab, Mar. Pollut. Bull. 133, 191-208 (2018).
(7) J.V. Gulmine, P.R. Janissek, H.M. Heise, and L. Akcelrud, Polym. Degrad. Stab. 79, 385-397 (2003).
(8) H. Lamnii, M. Nait-Abdelaziz, G. Ayoub, J-M Gloaguen, U. Maschke, and B. Mansoor, MATEC Web Conf. 165, 1-6 (2018).
(9) P. Gijsman, G. Meijers and G. Vitarelli, Polym. Degrad. Stab. 65(3), 433-441 (1999).
(10) A. Tidjani, Polym. Degrad. Stab. 68(3), 465-469 (2000).
(11) T. Åahin, T. Sinmazcelik, and Å. Åahin, Mater. Des. 28, 2303-2309 (2007).
(12) N. Lucas, C. Bienaime, C. Belloy, M. Queneudec, F. Silvestre, and JE Nava-Saucedo, Chemosphere, 73, 429-442 (2008).
(13) B. Gewert, M.M. Plassmann and M. MacLeod, Environ. Sci.: Processes Impacts, 17, 1513-1521 (2015).
(14) G. Everaert, L. van Cauwenberghe, M. de Tijcke, A.A. Koelmans, J. Mees, M. Vandegehuchte and C.R. Janssen, Environ. Pollut. 242(B), 1930-1938 (2018).
(15) G. Peng, P. Xu, B. Zhu, M. Bai and D. Li, Environ. Pollut. 234, 448-456 (2018).
(16) H.A. Nel, T. Dalu and R.J. Wasserman, Sci. Total Environ. 612, 950-956 (2018).
(17) E. Pagter, J. Frias and R. Nash, Mar. Pollut. Bull. 135, 932-940 (2018).
(18) L. Zada, H.A. Leslie, A.D. Wethaak, G.H. Tinnevelt, J.J. Jansen, J.F. de Boer and F. Ariese, J. Raman Spectrosc. 48, 1136-1144 (2018).
(19) A. Vianello, A. Boldrin, P. Guerriero, V. Moschino, R. Rella, A. Sturaro and L. Da Ros, Estuarine, Coastal Shelf Sci. 130, 54-61 (2013).
(20) S. Ziajahromi, A. Kumar, P.A. Neale and F.D.L. Leusch, Environ. Pollut. 236, 425-431 (2018).
(21) M. Claessens, S. De Meester, L. Van Landuyt, K De Clerck and C.R. Janssen, Mar. Pollut. Bull. 62, 2199-2204 (2011).
(22) M. Lares, M.C. Ncibi, M. Sillapanää and M. Sillapanää, Water Research, 133, 236-246 (2018).
(23) J. Li, H. Liu and J.P. Chen, Water Research, 137, 362-374 (2018).
Pik Leung Tang is with Agilent Technologies UK Ltd in Edinburgh, UK. Rick McCumskay, Mike Rogerson, Cath Waller, and Rodney Forster are with the School of Environmental Sciences at the University of Hull, in Hull, UK.

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.