Use of Raman Spectroscopy and 2D-COS for the Detection and Characterization of Chemical Interactions
Two-dimensional correlation spectroscopy (2D-COS) can reveal subtle chemical interactions that are difficult to discern when analyzing individual spectra. A demonstration of the subtleties of this technique can be seen in the analysis of ethanol–water and ethanol–methanol mixtures.
Raman spectroscopy, a laser-excited technique for measuring molecular vibrations, will be used to analyze ethanol–water and ethanol–methanol mixtures. It is well known that some solvent mixtures have a smaller volume than the sum of the volumes of the components, presumably a result of the strong hydrogen bonding. H-bonding is known to be one of the strongest noncovalent interactions, so we will examine mixtures of two solvents with H-bonded species. Initially, one would band-fit bands of vibrations to determine which functional groups are contributing to the interactions. But, because the Raman bands of the OH stretches where we expected to see effects are so broad, band-fitting can be problematic. This article will show how two-dimensional correlation spectroscopy (2D-COS) plots can reveal subtle changes that would be difficult to see in individual spectra.
Anyone who follows my columns or hears me talk at conferences knows that most of my work is done on a Raman microscope. When I accepted this job many years ago, Raman microscopy was an entirely new technology that offered totally new information for the scientist trying to understand the structure of new materials with engineered microstructures, or to solve manufacturing problems, or to understand processes like corrosion. My formal training was in physics, so I actually had to educate myself on the structure and spectroscopy of the materials that I was studying. But, given my background in physics, I understood immediately that the technology was capable of doing things that the chemists who invented the technology never considered. I understood that we could easily differentiate crystalline phases of organic and inorganic materials, and we could determine orientation. Because of my limited background in chemistry, I tended to stay away from purely chemical studies that involved a level of understanding of chemical structure, for which my background was weak. In this column, I am going outside my comfort zone to examine chemical solutions where it is known that there are chemical interactions. I am doing this as a result of several hardware and software innovations.
Because of the ubiquitous occurrence of fluorescence in many materials, it has become clear that using the 785 nm laser for excitation avoids such fluorescence in many, if not most, problematic samples, especially organic ones. In fact, inorganic materials may exhibit real fluorescence when excited at such a long wavelength because of the presence of transition metal and rare earth impurities, but I will not deal with that here. I have been aware of the phenomenon described in the preceding paragraph for a long time, so I thought it might be interesting to do a study of these mixtures on an instrument designed only for macro work (Macro RAM, Horiba Scientific, Edison, NJ). However, there is a major problem in such a study. When exciting at 785 nm, the detector, which is based on optical absorption of silicon, sees diminishing sensitivity as one scans farther out into the spectrum, because, as we approach the indirect bandgap, the capacity for absorption decreases. It is possible to correct for decreasing sensitivity, but only as long as the silicon is absorbing some light. Its bandgap, beyond which it will not absorb at all, is about 1.05 µm (3200 cm-1), which places it right in the middle of the OH region of the spectrum. At one point, I thought I would be clever and attempted to reconstruct the latter part of the OH spectrum, which goes out to 3600 cm-1, by doing a correction, but found that I couldn't make it work. What has recently become interesting is the introduction of a dual wavelength laser from IPS which easily switches between 785 nm and 680 nm. Exciting at 680 nm places the CH, NH, and OH part of the Raman spectrum at the same wavelength region as the fingerprint spectrum excited at 785 nm. So, I was able to record the full spectrum on an instrument with no moving parts, and with no sacrifice in spectral resolution.
The next part of my motivation was to use 2D-COS to analyze the spectra of mixtures with varying relative concentrations. What I was hoping to find was that I could visualize subtle changes in the OH region due to changes in H-bonding. I was disappointed to see that, in fact, there is not much evidence for changes in the OH envelopes. I did, however, observe other subtle changes that I did not expect to see, and you will see what I am talking about as you read further. For those of you who need an introduction to 2D-COS, I recommend the text by Noda and Ozaki (1).
Description of Samples
I have collected spectra from two sample sets, the first being an ethanol–water mixture, and the second an ethanol–methanol mixture. The ethanol–water mixture is a known azeotrope, which means that distillation cannot separate the two completely. The strong hydrogen bonding creates a 95.5% mixture that will distill without separation. In both sets of samples, I expected there to be strong H-bonding. The goal of this exercise is to determine how the H-bonding affects the spectra. Table I shows the composition of the mixtures that were used for this study. Since the dilutions were the same, the table labels two compounds as A and B, where A and B mean either ethanol and water or ethanol and methanol.
In my naïve understanding of chemistry from the point of view of a physicist, I would have thought that methanol would behave the same way as ethanol in a water–alcohol mixture. But, in fact, methanol does not form an azeotrope with water. It apparently does not form an azeotrope with ethanol, either. My question was whether the Raman spectra would provide any insight into these different behaviors.
Ethanol–Water System
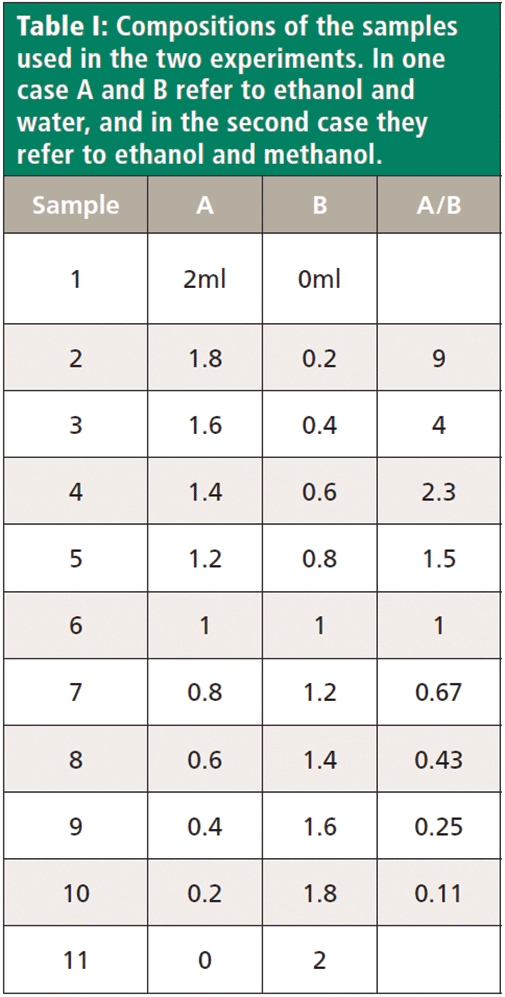
Figure 1 shows that Raman spectra of water and ethanol taken on the macro Raman instrument with the 785 and 680 nm laser combination.
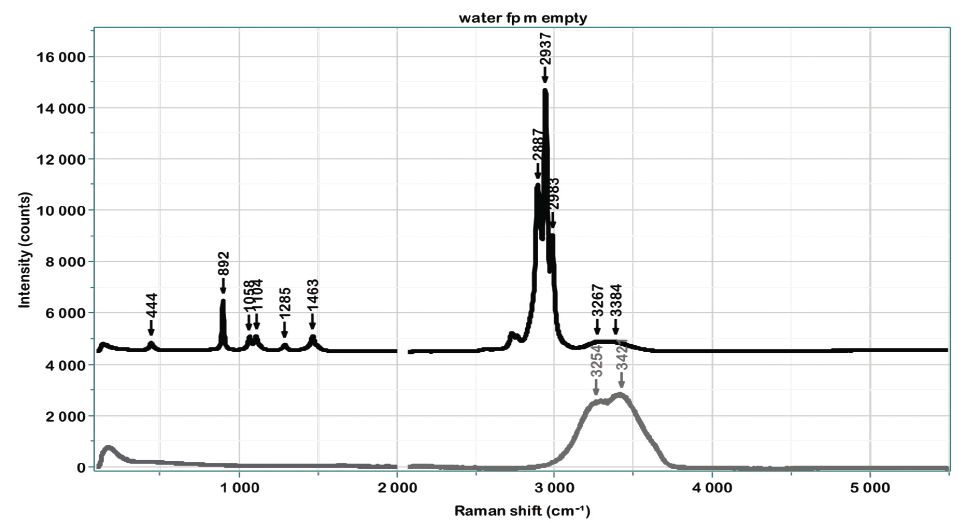
Figure 1: Raman spectrum of water (grey) and ethanol (black) in the fingerprint region (left, below 2000 cm-1) excited by 785 nm, and in the CH, NH, and OH region (right, above 2000 cm-1) excited at 660 nm.
The black spectrum is that of ethanol, and the grey that of water. All the sharp bands are attributed to vibrations of the ethanol molecule. The broad low frequency band in both samples is related to the speed of sound in the fluid, and, even though this spectral feature is truncated by the edge filter, it is seen to be different in the two liquids. Water also has an H-O-H deformation near 1640 cm-1, which is very weak because of the decreasing sensitivity of the charge-coupled device (CCD) detector, and which will not appear in any alcohol. In the high frequency part of the spectrum (now excited by 680 nm), there is an OH stretching envelope for both water and ethanol. In the water spectrum, however, the intensity extends farther in frequency shift, and if one assigns "peaks," it is clear that they are different.
Figure 2 shows the 2D-COS results for the 11 samples in the fingerprint region. The major changes will be the increasing intensity of the ethanol bands as the ethanol concentration is increased, and that is demonstrated clearly in the synchronous plots. In the lower left corner of the asynchronous plot, you can see evidence of the decreasing low frequency intensity of the ethanol band as the water band increases. In addition, there are more subtle changes occurring in the fingerprint region, which will become more evident when the plot is expanded.
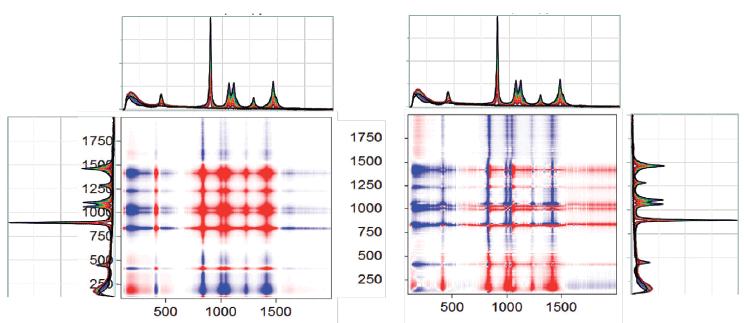
Figure 2: 2D-COS spectra, synchronous on the left and asynchronous on the right.
Figure 3 shows the synchronous and asynchronous plots between 850 and 1650 cm-1: in the upper right hand corner of the figure is the region around the intense band near 900 cm-1 of the asynchronous plot expanded for clarification. What you can see is clear evidence for a downshift of the ethanol band as the concentration of water is increased. While it is tempting to argue that these plots indicate a systematic, continuous frequency shift, analogous to what we see in solid materials, it is more likely that there are two (or more) closely spaced bands whose relative intensities are changing with composition (2).
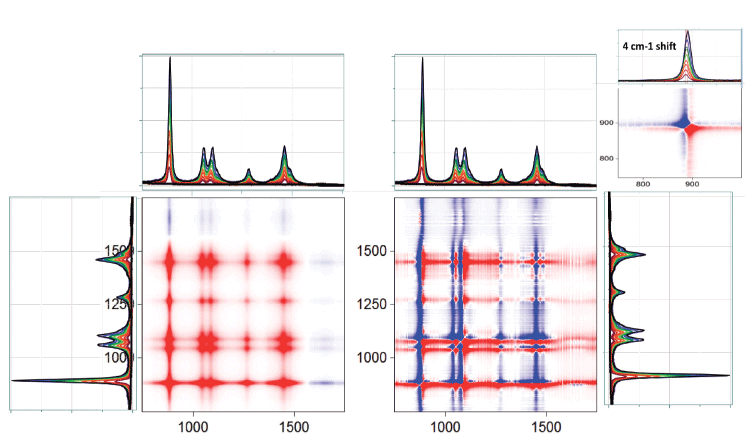
Figure 3: 2D-COS spectra as in Figure 2 but expanded between 850 and 1650 cm-1. The pattern in the lower left corner is expanded to show evidence of a change in intensity and an apparent peak shift.
Further expansion of this region shows shifts in almost all of the ethanol vibrational features, and, in the 1050 to 1100 cm-1 doublet, there even appears to be a third band that is not obvious from a visual inspection of the spectra. In addition, because some of the bands apparently shift down as the ethanol concentration decreases, and some shift up, you can eliminate the possibility of an artifact from an unstable wavenumber calibration. For sure, inspection of the literature can provide information on the band assignments as well as bond order and angles in ethanol. With this information, you can then use the shifts to determine how the interactions with the water are affecting the molecular configuration or bonding of the ethanol, or both.
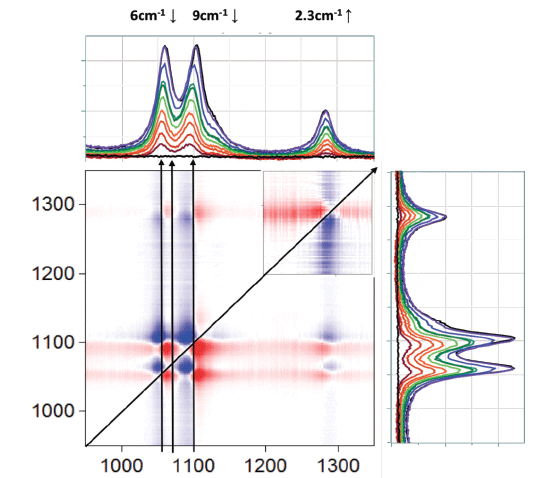
Figure 4 : 2D-COS asynchronous plot between 950 and 1350 cm-1.
Figure 5 shows the high frequency part of the spectrum that covers the CH, NH, and OH region. As expected in the synchronous plot, the bands between 2700 and 3000 cm-1 are increasing with the ethanol concentration, and the off-diagonal stripes indicate that the OH signal is decreasing in the opposite direction. At first, this last observation is a bit puzzling, because the water and the ethanol both have OH functional groups. The reason for this observation is that the OH signal in the water is higher than that from the ethanol. Expanding the asynchronous plot in the CH region in Figure 6 indicates the possible presence of four null points along the diagonal. What is curious is that these null points do not always superimpose on the spectral peaks. But note also that we really only see three spectral peaks, so, again, we consider the possibility that 2D-COS indicates the presence of unresolved peaks. The fact that the nodes do not coincide with the peaks is simply a result of the 2D-COS signal indicating changes, not absolute signals.
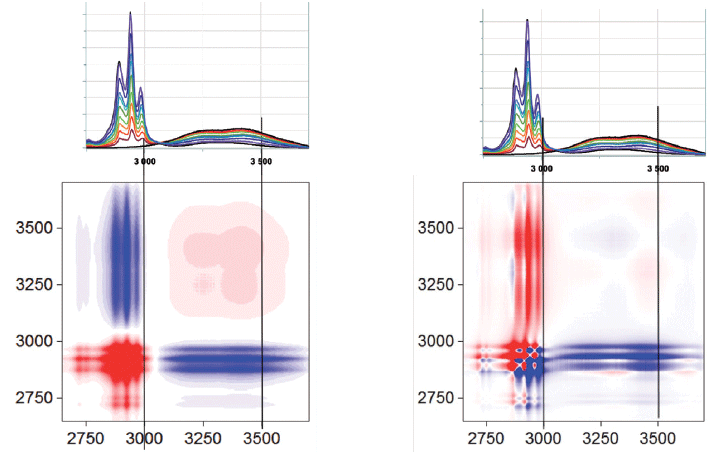
Figure 5: The 2D-COS synchronous and asynchronous plots of ethanol and water mixtures in the CH, NH, and OH region.
Figure 7 shows the 2D-COS results of the OH region of the spectrum. Because the OH intensity in the water is higher than in the ethanol, the higher frequency lobe that is assigned to water is increasing; the asynchronous plot shows a redistribution of intensity as the concentration of water is changed.
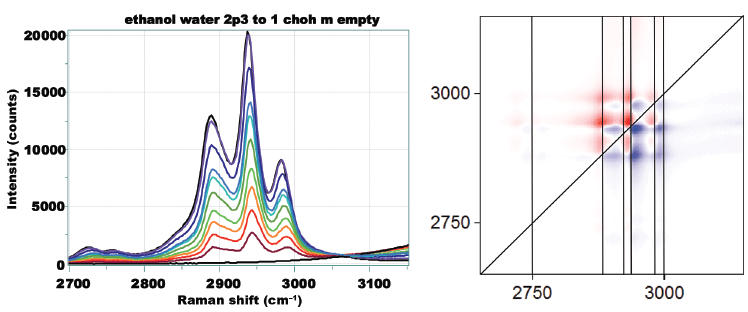
Figure 6: Asynchronous plots of CH region of the ethanol and water mixture.
Ethanol-Methanol System
Figure 8 shows the spectra of ethanol and methanol acquired on the macro Raman instrument with the two laser wavelengths. Looking at these individual spectra enables us to start to predict where to look for effects.
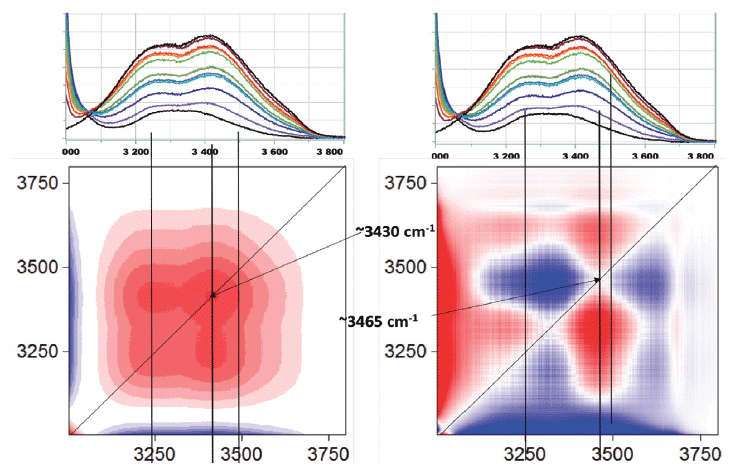
Figure 7: 2D-COS of the OH region of the ethanol and water mixture.
Figure 9 shows the 2D-COS results for the region between 850 and 1150 cm-1. Again, the asynchronous plot is where the most information can be seen. The ethanol band, which is fairly isolated in the spectrum, shows similar behavior in the asynchronous plot to what we saw in the ethanol-water system: an apparent peak shift with changing concentration. The more complicated pattern in the upper right hand quadrant is due to the fact that there are overlapping bands of the two species. The asynchronous plot in the low frequency region, shown in Figure 10, also shows a pattern that indicates peak shift with concentration. Figure 11 shows the asynchronous plot in the CH, and OH region. There is no intensity in the OH region, but peak shifting behaviors in the CH stretching bands.
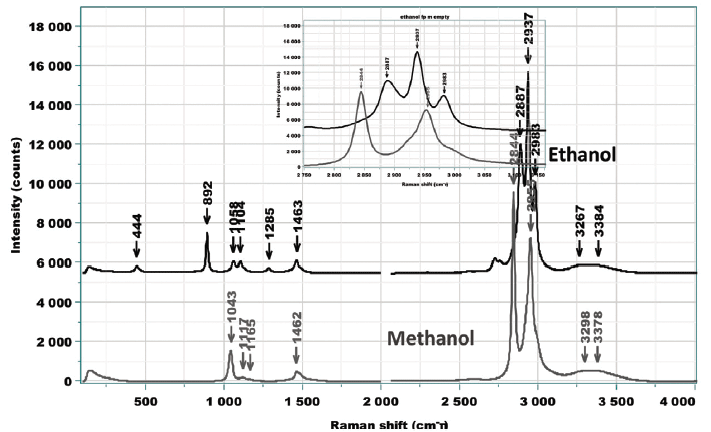
Figure 8: Raman spectra of ethanol and methanol acquired on the macro Raman instrument with the two laser wavelengths.
Discussion
While working on this project, I discussed with my teacher-mentor, Professor Isao Noda, what I was doing, and, to my surprise, he sent me several articles that he and others had done on similar systems (3,4). Again, I am reminded that there is nothing new under the sun (5). But there is much to be learned from these publications. For a start, my ethanol and methanol samples for sure had water in them, because I did not have the capability to distill and dry them before mixing them, as had these authors. The presence of some water may account for some differences between my results and those in the publications; compare Figure 9 above to Figure 4 in reference 2. Quoting from the abstract in this publication, "separate methanol and ethanol clusters are formed without heterohydrogen bonding between different alcohol species." This conclusion resulted from a more rigorous analysis of their data: preliminary PCA application demonstrated the existence of four separate regimes in concentration space. When the samples in these regimes were analyzed separately by 2D-COS, they suggested the existence of molecular interactions and the formation of rings and chains. Therefore, even though my samples were not prepared as rigorously as they should be for such experiments, these previous publications also show that it is not the OH groups that are showing the effects of molecular interactions in the mixtures.
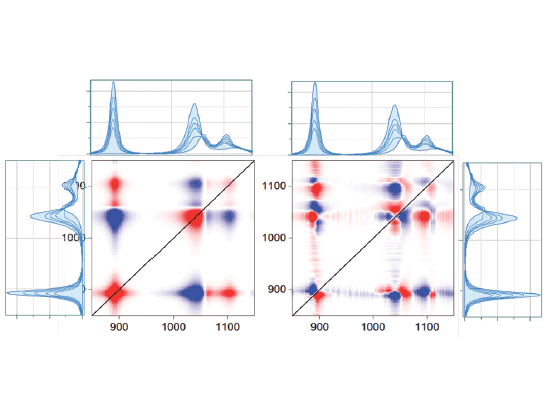
Figure 9: 2D-COS between 850 and 1150 cm-1.
Conclusions
This small project is another example of how 2D-COS enables the use of vibrational spectroscopic data to elucidate the chemical state of a system. I am, in no way, an expert in its application, but I am intrigued by its possibilities, which is why I have applied it in several of my columns in the past year. This journal, in fact, provides me an opportunity to relate my thought processes in getting to use this new technology, and consequently, Professor Noda has recommended that others who are interested in learning to apply 2D-COS may be able to take advantage of my learning experiences to start to use it themselves.
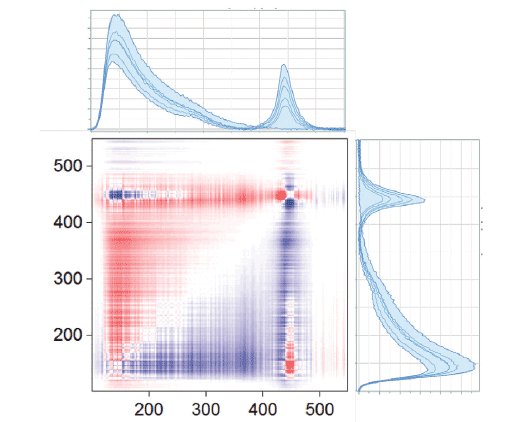
Figure 10: Asynchronous plot of ethanol and methanol mixtures in the low frequency part of the spectrum.
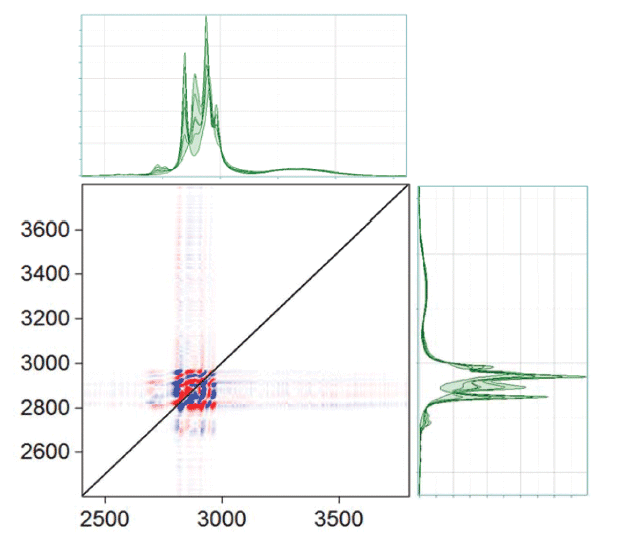
Figure 11: Asynchronous plot of ethanol and methanol mixtures in the CH, OH part of the spectrum.
References
(1) I. Noda and Y. Ozaki, Two-Dimensional Correlation Spectroscopy (Wiley & Sons, Chichester, West Sussex, UK, 2004).
(2) A discussion that I had with Professor Noda indicates how far I am from my area of expertise in this work. He reports that while many publications discuss the butterfly behavior in the asynchronous plots as evidence for a continuous peak shift, he says that, at least in liquids, these publications show simulated plots and do not represent the reality in liquid systems. He invokes Lippincott's theory of "distributed populations of discrete and specific associated species" rather than Pimentel's model in which "the peak position shift of a hydrogen-bonded system is dependent on the elongation of the hydrogen bond....which created the foundation for the 'band frequency theory.'" The various closely spaced bands that would account for the apparent peak shift he assigns to distinct clusters formed in H-bonded systems.
(3) C. Mello, T. Mello, E. Severi, L. Coelho, D. Ribeiro, A. Marangoni, R. J. Poppi, and I. Noda, J. Chem. Phys. 131, 084501 (2009).
(4) P. Tornaz, W. Wrzeszcz, S. Mazurek, R. Szostak, and M.A. Czarnecki, Spectrochim. Acta, Part A 197, 88-94 (2018).
(5) Ecclesiastes 1:9
Fran Adar

Fran Adar is the Principal Raman Applications Scientist for Horiba Scientific in Edison, New Jersey. Direct correspondence to: SpectroscopyEdit@UBM.com.

A Proposal for the Origin of the Near-Ubiquitous Fluorescence in Raman Spectra
February 14th 2025In this column, I describe what I believe may be the origin of this fluorescence emission and support my conjecture with some measurements of polycyclic aromatic hydrocarbons (PAHs). Understanding the origin of these interfering backgrounds may enable you to design experiments with less interference, avoid the laser illuminations that make things worse, or both.
Raman Microscopy for Characterizing Defects in SiC
January 2nd 2025Because there is a different Raman signature for each of the polymorphs as well as the contaminants, Raman microscopy is an ideal tool for analyzing the structure of these materials as well as identifying possible contaminants that would also interfere with performance.