HPLC–MS Characterization of 99.5% Pure Compounds Using Miniaturized Mass Detector
Special Issues
Detecting impurities in any chemical reaction is becoming increasingly important to detect those present at low levels (for example, 0.5%).
Detecting impurities in any chemical reaction is becoming increasingly important to detect those present at low levels (for example, 0.5%). Common methods of assessing purity include chromatography with a detector, such as an ultraviolet (UV) light detector, or an evaporative light scattering detector (ELSD). However, these detectors do not give any information about the molecular weight or structure of the impurities detected and thus make identification difficult.
A complementary detector, which can be used with a chromatograph, is the mass spectrometer (MS). Traditional MS systems available are too bulky, expensive to purchase and run, and power hungry for use in every laboratory. These limitations have lead to compromises in the choice of chromatography detectors that can be used in synthetic laboratories.
The Microsaic 4000 MiD® gives the chemist the ability to have a simple-to-use, low cost mass detector at the bench. The system is portable and modular so it can be easily integrated into a wide range of bench-top applications.
Here we report on how the Microsaic 4000 MiD® can be used as an HPLC detector to identify the presence of impurities that would otherwise be lost using popular detection methods.
Methods
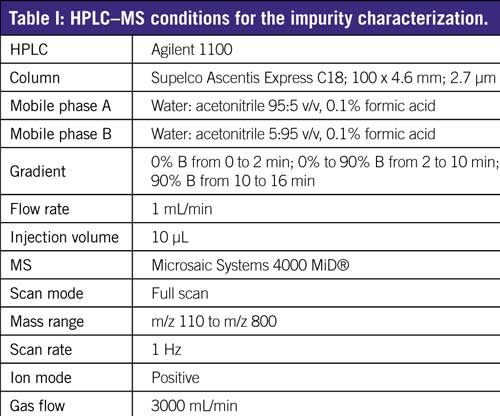
Table I show the HPLC–MS conditions used for the impurity characterization.
Test samples were prepared, with the major component at 1 mg/mL and the minor one at 5 µg/mL (0.5% w/w). Two samples were prepared. Test sample A was made up by mixing Verapamil at 1 mg/mL and Bromopride at 5 µg/mL (0.5% w/w). Test sample B was Cortisone (1 mg/mL) and Verapamil (5 µg/mL–0.5% w/w).
Results and Discussion
Well Resolved Impurities
Test sample A contains two well resolved compounds, Bromopride and Verapamil, with the minor component eluting first. Both compounds are visible in the total ion chromatogram (TIC) shown in Figure 1. The mass spectra of the two chromatographic peaks confirmed the presence of bromopride and verapamil with m/z 344.4 and m/z 455.4, respectively.
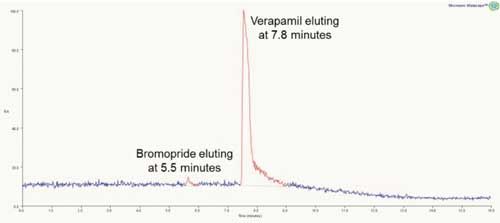
Figure 1: Total ion chromatogram for test sample A.
Co-Eluting Impurities
Test sample B is an example of two compounds that coelute and could not be distinguished by UV only. Figure 2 shows the TIC and UV data of the cortisone–verapamil test mix. As expected, this shows a single major UV and TIC peak. When analyzing the TIC, mass spectra data are dominated by the cortisone ion, but the one from the tail of the peak shows an ion corresponding to verapamil.
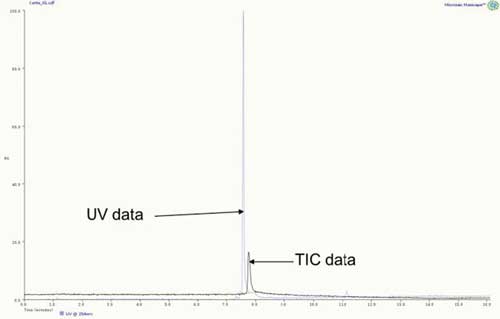
Figure 2: Combined total ion and UV chromatograms of test sample B.
Conclusions
The Microsaic 4000 MiD® coupled to a HPLC enabled the identification of unknown impurities down to the low level of 0.5% (w/w) in full scanning mode. In the case of coelution on the HPLC system, the 4000 MiD® could help to identify an impurity where the UV data would provide misleading results.
Microsaic Systems plc
GMS House, Boundary Road, Woking, Surrey, UK
