ICP-MS Analysis of Noble Metals at Low Levels in Geological Reference Materials and Ores
Special Issues
This study reveals the potential of triple-quadrupole ICP-MS for reliable quantification of noble metals at ultratrace levels even in difficult matrices.The effective use of reactive gases for interference removal and a thorough and effective protocol for sample preparation and handling are essential.
In most cases, elements such as gold, platinum, rhodium, and iridium, generally considered noble metals and partly belonging to the so-called platinum group metals (PGMs), are considered interference free in inductively coupled plasma–mass spectrometry (ICP-MS). However, in cases where they need to be accurately quantified at low levels, for example, to determine if their concentration in an ore deposits is high enough to economically justify mining and processing the ore, other elements may easily interfere and cause significant bias. Even trace amounts of hafnium and tantalum severely affect the detection of Pt and Au as a result of interference from HfO, HfOH, and TaO, and negatively impact analysis results. Especially when very low amounts need to be detected, kinetic energy discrimination (KED), the primary tool applied to remove polyatomic interferences in single-quadrupole ICP-MS, is not sufficiently effective to eliminate all interferences at the level required for the accurate quantification of ultratrace amounts of Au, Pt, Rh, and Ir.
The so-called platinum group metals (PGMs) strictly consist of six precious metals: ruthenium (Ru), rhodium (Rh), palladium (Pd), osmium (Os), iridium (Ir), and platinum (Pt). All of the metals possess unique characteristics and are important for applications such as catalysts, both in the chemical industry as well as in catalytic converters in cars, jewelry, and electronic components. Because of their use as catalysts in synthesis for core chemicals for polymerization, they may also be found in consumer products made from silicone or other plasticware. They are also mentioned as potentially occurring impurities in pharmaceutical products, and must be tested for if evidence for their presence in a particular product exists. Gold is another element of great interest. In nature, noble metals occur under certain geological conditions and in certain geographic regions (for example, South Africa, but also Russia or the northern area of the American continent, Canada, and Alaska). Noble metal ores may contain varying amounts and composition of metals at relatively low levels (up to 10 g per ton). Therefore, a careful characterization of all materials is required to make a final decision whether a source is viable to be explored. Because of their chemical nature, sample preparation is often lengthy and involves many different steps to reliably extract the noble metals into solution. Hence, their final concentration in a solution measurable with atomic spectroscopy techniques such as inductively coupled plasma–optical emission spectroscopy (ICP-OES) or ICP-mass spectrometry (MS) is low.
Although higher mass regions in ICP-MS are not affected by the most abundant Ar-based polyatomic interferences, certain elements may be present in a sample that lead to significant false positive contributions on elements such as Rh or Pt. Whereas the detection of monoisotopic 103Rh may be affected through 206Pb2+ or different polyatomic interferences such as 87Sr16O+ or 63Cu40Ar+, the interferences on the main isotopes of Pt and Au are mainly caused by the presence of Hf in a sample, forming HfO, HfOH, and TaO interfering ions.
In this article, interference-free analysis of several noble metals will be demonstrated in two sample matrices. Method development was accomplished using a rock sample that did not contain the target elements, leading to excellent detection and quantification limits and quantitative spike recoveries. Finally, the method was applied to an ore sample containing the elements of interest, and the results demonstrate that interference-free analysis is achieved.
Experimental
All measurements were accomplished using a Thermo Scientific iCAP TQ ICP-MS system (Thermo Fisher Scientific). The selection of analytes, appropriate analysis mode (single quadrupole vs. triple quadrupole, choice of reactive gas) was accomplished using the Reaction Finder method development assistant software. Further analysis modes for particular isotopes were added manually for comparison. The mass selection in the first quadrupole was controlled using intelligent mass selection (iMS) in all cases. The instrument was tuned daily using the autotune routines supplied with the operating software. To compare the results obtained using different measurement modes, the ICP-MS system was configured to scan all samples in all measurement modes. An overview of the instrument´s settings can be found in Table I. Two certified reference materials were prepared for analysis, GSP 2 (United States Geological Survey), a granodiorite, and AMIS 0416 (African Mineral Standards), a platinum ore. All samples were prepared externally following a previously published protocol (1). Before analysis, all digests were diluted 20 times using a 3% hydrochloric acid, 1% nitric acid diluent and an internal standard solution (containing In and Bi at a concentration of 1 ng/mLeach) was added. After calibration using different multielement standard solutions (concentration range between 50 and 1000 ng/L, diluted in the same acid combination), an initial calibration verification (ICV) was performed (50 ng/L of different PGMs). During analysis, repeated continuing calibration verification checks (CCVs) were interspersed periodically at a concentration level of 500 ng/L. To verify the accuracy and precision of the proposed method, GSP 2 was spiked with trace amounts of different noble metals (10 and 25 ng/L) and analyzed as a quality control check repeatedly during analysis.
Results and Discussion
In a first step, suitable measurement conditions involving different scan modes (single quadrupole vs. triple quadrupole) and reactive gases (no gas, KED, O2, or NH3) were tested for the different PGMs and gold. To perform this study in a realistic sample matrix, containing typically interfering elements plus a considerable matrix load, the GSP 2 reference material provided by the United States Geological Survey (USGS) was chosen. This material represents a granodiorite rock collected in Silver Plume, Colorado. Most importantly for this study, GSP 2 does not contain significant amounts of any of the elements under scrutiny in this study, but plenty of interfering elements such as Hf (information value 14 ± 1 µg/g, interfering on Pt), Lu and Yb (0.23 ± 0.03 and 1.6 ± 0.2 µg/g respectively, interfering on Ir), Cu and Sr (43 ± 4 and 240 ± 10 µg/g respectively, interfering on Rh), among others. The major components (and hence the sample matrix) consists of SiO2 (66.6 ± 0.8 wt%), Al2O3 (14.9 ± 0.2 wt%), Fe2O3 (4.90 ± 0.16 wt%), and K2O (5.38 ± 0.14 wt%).
To eliminate the overlaps caused by the interfering elements and facilitate the detection of elements such as Rh, Pd, Ir, Pt, and Au at ultratrace levels, different measurement modes were applied. For example, Pt can be analyzed using two different reactive gases, O2 and NH3. Figure 1 highlights the reaction pathways in the collision–reaction cell. Because no reaction occurs between Pt and O2, it will pass through the cell unaffected. In contrast, the interference, HfO, initially having the same mass-to-charge ratio, is converted into HfO2 and hence eliminated in the analyzing quadrupole (Q3). At the same time, the first quadrupole (Q1) filters out all other ions with lower and higher mass, which could potentially react in a similar way, and hence create other interferences on the target ion mass. With ammonia, Pt undergoes different reactions forming Pt-NH3 clusters, the most abundant product ion being Pt(NH3)2, thus leading to a mass shift of 34 amu. Again, the HfO interferences will not react in the same way and will be eliminated in the analyzing quadrupole. Similar reaction schemes can also be applied for other PGMs, such as Rh, Pd, Ir and also Au. In most cases, they are not reactive toward O2, whereas their interferences can be efficiently eliminated.
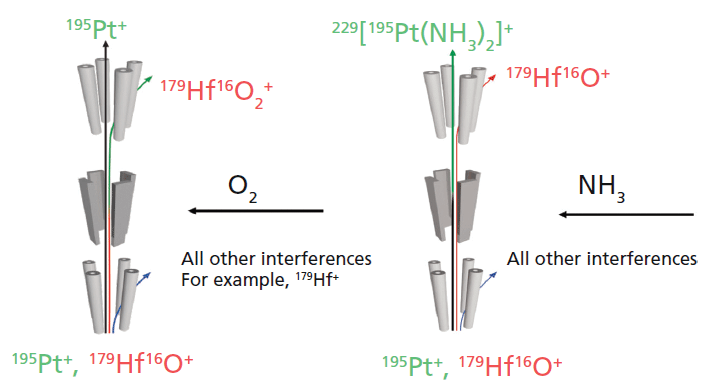
Figure 1: Schematic illustrating the analysis of platinum using triple-quadrupole ICP-MS operated with oxygen and ammonia as reaction gas.
To prove the successful elimination of interferences and consistent quantification at ultratrace levels, GSP 2 was analyzed unspiked, but also with low-level spikes (10 and 25 ng/L). This approach allows determination of the blank equivalent concentration for each of the elements under study to ensure complete interference removal, and also verification of the capability to accurately detect at these concentration levels. In total, GSP 2 and both spiked samples were analyzed six times in an 11-h analysis. The results for 103Rh and 195Pt are summarized in Table II. The signal observed in the unspiked solution of GSP 2 was consistently found to be around 5 ng/L, so that probably a very low amount of Pt is indeed present in the material. Similar results were obtained for other elements (data not shown). Typical instrumental detection limits were observed to be between 0.1 and 2 ng/L for all elements investigated in this study.
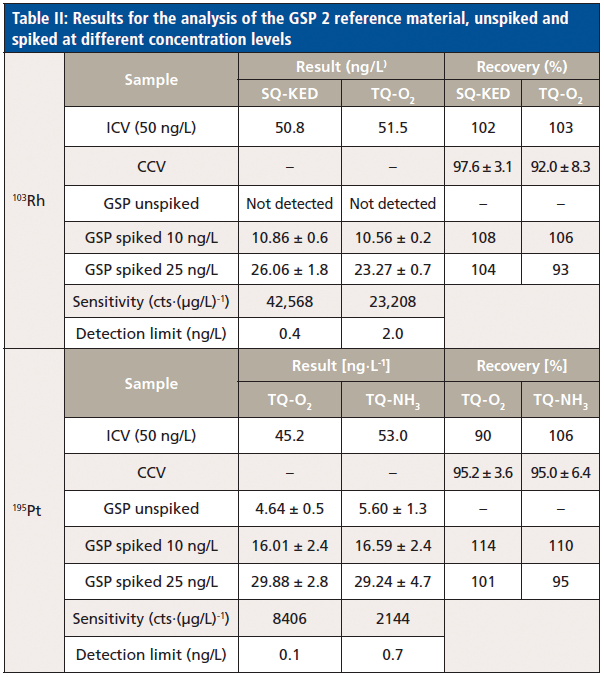
As can be seen from the results obtained for the unspiked samples, triple quadrupole–based modes allow complete removal of potential interferences. To additionally prove the effectiveness of the selected modes for interference removal the sample was spiked with the main interfering elements (Sr, Hf, Ta, and Lu) at the level equivalent to their initial concentration in the sample, and the increase of the background equivalent concentrations (BECs) for the noble metals was evaluated to be negligible. At the same time, all tested measurement modes accurately and precisely recovered the spiked amounts of both elements. Despite a lower detection sensitivity in comparison to typical measurement modes applied with single-quadrupole ICP-MS system (no gas or kinetic energy discrimination), the attainable limits of detection are still in the single digit nanogram-per-liter range, or even lower.
In the same analysis sequence, another certified reference material, AMIS 0416, was analyzed to validate the method further. This material was derived from a platinum ore obtained in the Bushfeld complex in South Africa and is certified for its content of different noble metals. Its main composition is slightly different from GSP 2, containing much higher amounts of Fe2O3 (22.71 ± 0.26%), but also Cr2O3 (28.18 ± 0.42%).
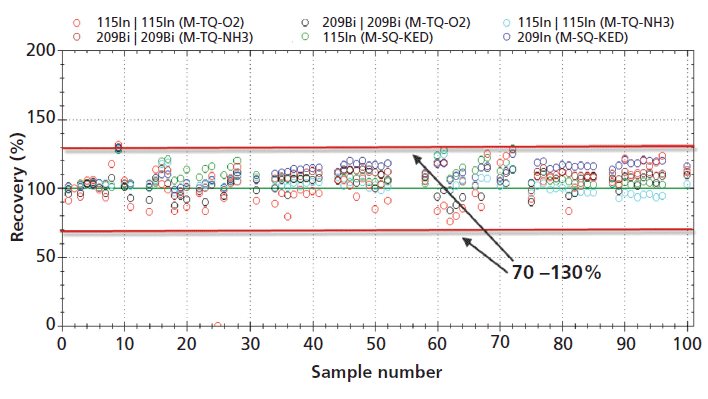
Figure 2 shows the internal standard recovery for both internal standards in all measurement modes over the course of an 11-h analysis sequence, containing in total more than 100 samples. As can be seen, the different sample matrices (acid mixture used to dilute standards and QC checks, GSP 2 and AMIS 0416 reference materials) do not significantly affect the internal standard recovery over time, so that also the analysis of a high number of samples is feasible without interruptions through failed QCs or excessive signal suppression (less than ±30% as widely accepted in different regulatory guidelines).
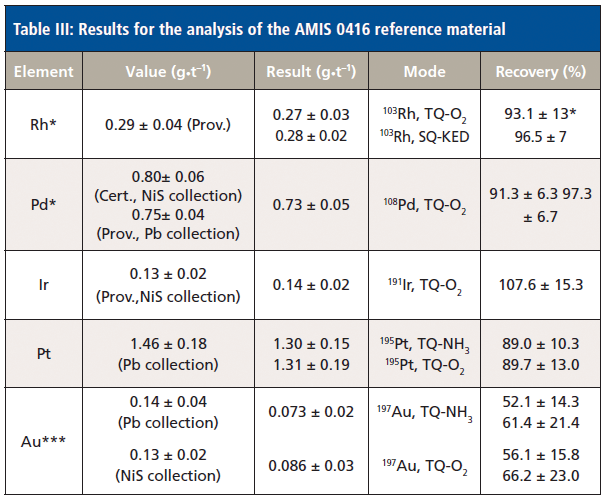
The results obtained for the analysis of AMIS 0416 are displayed in Table III. This table also contains all relevant information about the certified and provisional values as supplied by the certificate of analysis, as well as the method for collection (NiS vs. Pb collection), where applicable. Results are reported separately for the different measurement modes that were evaluated in this study. The given recoveries are either calculated for different measurement modes vs. available reference value when more than one result was available (for example, 103Rh), or relative to the reference values for different collection assays when more than one reference value was available (for example, 108Pd). In all cases, the concentrations in the finally measured solution were fairly low (between 20 to 350 ng/L), so that complete elimination of false positives was key to avoid large scaling errors when correcting for the dilution factor. As can be seen, the results for the platinum group elements Rh, Pd, Ir and Pt agree well with the certified values. However, only the result for Au falls short with respect to the reference value. Most likely, as results obtained with GSP 2 indicate excellent recoveries, this shortfall is related to an unresolved issue during sample preparation or handling, which may lead to a loss of Au in the analyzed samples. Gold is known to be ideally stabilized with higher concentrations of hydrochloric acid, typically above what was used as the acid diluent in this study.
Conclusions
This study reveals the potential of triple-quadrupole ICP-MS for comprehensively eliminating interferences and subsequently allowing reliable quantification of noble metals at ultratrace levels, even in difficult matrices. The effective use of reactive gases for interference removal, complemented by mass filtration before the collision–reaction cell enables full elimination of interferences of different nature (polyatomic, isobaric, or doubly charged) and is therefore a significant improvement for challenging sample matrices such as rocks or ores. However, whereas controlling the analysis is one part of the story, a thorough and effective protocol for sample preparation and handling is equally important for successful ultratrace analysis of noble metals given their similar, yet slightly different chemical behaviors.
References
(1) V.K. Karandashev, V. Khvostikov, S.V. Nosenko, and Zh.P. Burmii, Inorganic Materials 53, 1432–1441(2017).
Daniel Kutscher and Shona McSheehy Ducos are with Thermo Fisher Scientific, Bremen, Germany. Alexey Leykin is with Intertech Corp., Moscow, Russia. Simon Nelms is with Thermo Fisher Scientific, Hempstead, United Kingdom. Direct correspondence to: daniel.kutscher@thermofisher.com

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.