Improving on GC–MS Performance for Demanding Applications: The Use of GC Coupled to Tandem-Quadrupole MS
Special Issues
Gas chromatography–mass spectrometry (GC–MS) and liquid chromatography (LC)–MS are widespread successful approaches, based on single-quadrupole MS, for the routine detection, identification, and quantitation of compounds. There has, however, been increasing interest in the use of tandem MS in more challenging, complex matrices such as those commonly found in food, environmental, and biological analyses. The combination of GC with tandem-quadrupole MS (MS-MS) is discussed, where the inherent increase in selectivity and sensitivity of the approach has enabled rapid, confident compound detection and quantitation for such demanding applications.
Gas chromatography–mass spectrometry (GC–MS) and liquid chromatography (LC)–MS are widespread successful approaches, based on single-quadrupole MS, for the routine detection, identification, and quantitation of compounds. There has, however, been increasing interest in the use of tandem MS in more challenging, complex matrices such as those commonly found in food, environmental, and biological analyses. The combination of GC with tandem-quadrupole MS (MS-MS) is discussed, where the inherent increase in selectivity and sensitivity of the approach has enabled rapid, confident compound detection and quantitation for such demanding applications.
Over the last decade, tandem mass spectrometry (MS-MS) techniques have become firmly established in laboratories supporting a wide range of activities, including the life sciences, pharmaceutical, clinical, food and beverage, environmental, petrochemical, and chemical materials industries. Both liquid chromatography (LC) and gas chromatography (GC) have been used successfully as inlets for MS-MS systems, and the technique has become the dominant workhorse for quantitative LC–MS-MS in many laboratories. When used in combination, LC- and GC-based MS-MS are complementary and enable analysts to detect the widest possible range of compounds in a sample. This article presents GC coupled to tandem-quadrupole MS (GC–MS-MS), describing both the core technology and experimental modes of operation. Several applications will then be discussed, demonstrating the ability of GC–MS-MS to provide effective solutions for the analytical challenges faced by many laboratories.
MS-MS techniques require the use of two mass analyzers in series or a single analyzer that can be used sequentially, such as an ion trap, to analyze precursor and product ions (1). The mechanism of MS-MS involves mass selection of a precursor ion in the first analyzer, fragmentation of the precursor (typically using collisionally induced dissociation [CID]), and then mass analysis of the resulting product ions in the second analyzer. Operated in this way, MS-MS can provide useful structural information by establishing relationships between precursor ions and their fragmentation products, and it can achieve better selectivity and sensitivity for quantitative analysis.
Various combinations of analyzers have been investigated and many commercialized, including the use of magnetic sector, quadrupole, time-of-flight, and trap technologies (2,3). Originally, such instruments often were found only in specialist MS groups with high levels of expertise and substantial funding. However, developments in technology and demand from the market have resulted in vendors being able to offer lower cost, high-performance, benchtop MS-MS instruments. This newer generation of instruments has made MS-MS techniques accessible to many more laboratories, including those undertaking routine work, and has ensured the widespread adoption of the technology.
One of the most popular types of MS-MS instrument has been the triple-quadrupole mass spectrometer, first developed by Yost and Enke (4). The introduction of the first commercial triple-stage quadrupoles in the early 1980s greatly helped to popularize MS-MS, and today tandem-quadrupole MS-MS is one of the most widely used tandem techniques as a result of its lower relative cost, ease of use, and ability to carry out a wide range of MS-MS experiments.
GC–MS-MS Instrumentation
A schematic diagram of a GC–MS-MS tandem-quadrupole instrument is shown in Figure 1. The diagram shows a heated GC interface bringing eluent from the GC column into the MS source. Ionization is achieved under electron ionization (EI) or chemical ionization (CI) conditions and the EI and CI inner sources are interchanged readily without the need for venting the full system. The mass analyzer consists of two quadrupoles (labeled here as MS1 and MS2) separated by a hexapole collision cell. Tandem quadrupoles use two stages of mass analysis, the first to preselect an ion (the precursor ion) and the second to analyze fragments (product ions) induced by collision with an inert gas, usually argon, in the collision cell. The collision cell shown here is a linear hexapole, but other designs exist such as 90° and 180° curved cells. An off-axis photomultiplier detector is utilized in this schematic, but electron multipliers also are employed.
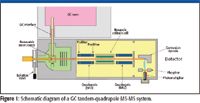
Figure 1
GC–MS-MS Experimental Modes
Tandem-quadrupole MS-MS offers great flexibility in the type of experiments that can be carried out on ions generated in the source. Figure 2 shows the schematic of the instrument ion optics and can be used to explain the different operating modes.
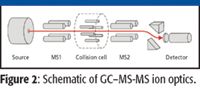
Figure 2
The first experiments we consider are the MS operating modes. In MS1 mode, the first quadrupole (MS1) is used as the mass filter with the collision cell and second quadrupole operated in transmission-only mode. (This is achieved by turning off the collision gas in the cell and using MS2 in what is known as Rf-only mode. Under these conditions, ions exiting MS1 will be transmitted directly through the cell and MS2 to the detector.) This mode provides the highest transmission and is directly analogous to using a single quadrupole mass spectrometer. It is possible to use the MS1 mode for acquiring both full scan spectra (by using MS1 in scanning mode), and selected ion monitoring (SIM) data (by using MS1 in static mode).
The ability to generate full scan spectra and SIM data is a useful feature of tandem-quadrupole MS, but the real benefits of the technique are realized fully only when the MS-MS operating modes are employed.

Table I: Summary of MS-MS operating modes
There are four common MS-MS operating modes, which are summarized in Table I and Figure 3. Product ion mode (Figure 3a) is the most commonly used MS-MS scanning mode and typically is used for structural elucidation and in method development for multiple reaction monitoring (MRM)-based studies, which are used for demanding quantitative applications. When developing MRM methods, product ion scanning experiments are used to identify the product ions for use in MRM transitions and to optimize CID tuning conditions to maximize the yield of a specific product ion to be used in MRM analysis, thereby improving the quantitative performance.

Figure 3
Figure 3b shows the precursor ion-scanning mode. This technique will enable all precursor ions that give rise to a specific product ion to be recorded. Typical applications, therefore, often involve the detection of compounds having closely related structures, which give rise to a common product ion. The benefit of the MS-MS experiment is that it filters out chemical noise and potentially interfering ions, greatly improving the sensitivity of detection in complex sample matrices and simplifying structural confirmation and elucidation.
The multiple reaction monitoring (MRM) mode (Figure 3c) is a highly selective MS-MS experiment and is the most commonly used mode in quantitative applications. Because both MS1 and MS2 are static, greater dwell time on the ions of interest is allowed; therefore, better sensitivity is achieved compared to scanning MS-MS. MRM mode provides very high selectivity if appropriate transitions are selected, and in combination with high sensitivity, this is an ideal technique for trace-level quantitation of target compounds in highly complex or dirty matrices.
The final MS-MS mode is constant neutral loss (or gain) (CNL), which is shown in Figure 3d. CNL detects the loss (or gain) of a specific neutral fragment or functional group from an unspecified precursor or precursors. Applications would include screening mixtures for a specific class of compound that is characterized by a common fragmentation pathway. The scans of MS1 and MS2 are synchronized. When MS1 transmits a specific parent ion, MS2 "looks" to see if that parent loses a fragment of a certain mass. If it does, it registers at the detector. The result is that spectrum shows the masses of all parents that actually lost a fragment of a certain mass.
Applications
Tandem quadrupole–based GC–MS-MS provides the widest possible range of MS-MS experimental modes, making it a powerful tool for both structural elucidation and targeted quantitative analysis. In practice, the most popular use of the technique, and that which has contributed most to its growth, has been the use of MRM mode for low-level quantitation of compounds in complex matrices. The following sections will focus on some of the specific application areas of significance and will demonstrate the benefits of the technology over conventional approaches.
Food Safety Applications
International trade in food products has increased rapidly in recent years (5), with many nations worldwide placing strict legislative demands on the quality of imported foods. Exporters are thus required to check the quality of products before they are shipped or risk rejection and even destruction of the shipment at considerable cost to the producer. The net result has been an increase in monitoring, much of which is for chemical residues at trace levels, requiring MS techniques. Two very extensively legislated, and therefore, commonly monitored, groups of residues are veterinary drugs and pesticides.
Pesticides
There are over 800 pesticides registered for use worldwide and there is an ever-increasing requirement to monitor levels of these compounds in foodstuffs. Given the wide range of pesticides and product types to be analyzed, the analytical challenge is to maximize the number of residues that can be determined, minimize the variety of methods required, keep run times short, and achieve limits of detection at or below the legislated reporting levels. For these reasons, multiresidue methods in which generic sample preparation procedures are employed have become very popular. Inherent to this approach is that cleanup of extracts is only possible to a very limited extent. When complex matrices such as baby food, spices, or tobacco are analyzed, enhanced selectivity in detection is required to make up for the low selectivity in sample preparation.

Figure 4
Traditionally, much pesticide analysis has been carried out by GC–MS using single quadrupole detection and SIM experiments. This can, however, prove limiting when more complex matrices are encountered. Figure 4 illustrates this by comparing the SIM and MRM data of the pesticide malathion in several matrices of varying complexity. Selecting appropriate conditions for an MRM involves choosing an appropriate characteristic precursor ion and optimizing the CID conditions to give suitable fragmentation and selection of product ions. This process is illustrated in Figure 5. Here we see the EI spectrum of methyl-pirimiphos and a product ion spectrum resulting from fragmentation of the m/z 305 molecular ion. Many applications require more than one transition to be used in order to confirm the presence of a target analyte, and so two MRM transitions are shown in Figure 5, enabling both quantitative and confirmatory analysis to be carried out in a single injection.

Figure 5
As discussed previously, the limited sample cleanup required by multiresidue methods can result in very complex sample matrices such as those shown in Figure 6a. The selectivity of GC–MS-MS in overcoming matrix interferences can enable over 100 residues to be determined in such matrices (Figure 6b). The ability to quantify and confirm pesticides, sometimes at levels below 0.01 mg/kg, is extremely challenging in such applications, but the sensitivity and selectivity of MRM mode can meet this challenge effectively.

Figure 6
Steroid Growth Promoters
A wide range of drugs, including hormones and antibiotics, are used in farm animals for therapeutic purposes but also by some farmers to alter the characteristics of the meat by improving growth performances. European countries have implemented screening programs to reduce the abuse of such drugs and to ensure that meat sold for human consumption is free from residues. However, concentration levels are ever-decreasing, and so traditional methods of detection will need to be improved to maintain efficient control of such substances within the food chain. Similar compounds are also used illegally to enhance performance in sport, and the analytical strategies adopted by the World Anti-Doping Agency (WADA) laboratories closely parallel those described here for food safety.
Steroid growth promoters often are analyzed by GC–MS with single quadrupoles, using selected ion monitoring (SIM). Results must satisfy the current EU legislation on confirmation criteria, Commission Decision 2002/657/EC, which stipulates that four structure-related ions for each analyte with the correct ion ratio must be monitored when using SIM mode. When the analysis requires the unambiguous identification of these compounds at trace concentrations from highly complex matrices, difficulties can be encountered. These are often interferences from co-eluting compounds, which appear on the SIR trace, making accurate quantification prone to error (Figure 7).
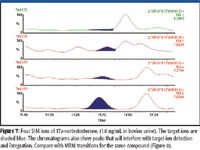
Figure 7
Selectivity can be gained through the use of the MRM technique. When MRM is used, legislation stipulates that monitoring of two structure-related transitions for each analyte suffices for confirmation of identity. Figure 8 clearly illustrates the advantage of MRM because the analyte at the same concentration and in the same extract is resolved from all matrix interferences and can be quantified easily and confirmed with improved signal-to-noise ratio (S/N).
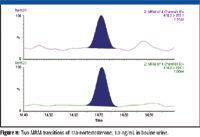
Figure 8
Environmental Application: Synthetic Pyrethroids in Wastewater
Synthetic pyrethroids were introduced as a less hazardous and less persistent alternative to organochlorine and organophosphate insecticides on the basis of their low acute toxicity to mammals. Although pyrethroids have a low acute toxicity to mammals, they are extremely toxic to aquatic organisms, including fish such as the bluegill and lake trout, with LC50 values less than 1 ppb (6). Two of the synthetic pyrethroids, cyfluthrin and permethrin, are listed in the Dangerous Substances Directive (76/464/EEC), which requires the reduction or elimination of discharges of certain substances to the environment. Under the directive, the environmental quality standard (EQS) for cyfluthrin is 0.001 μg/L and for permethrin is 0.01 μg/L.
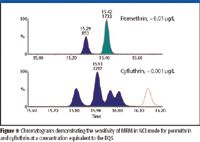
Figure 9
Analytical methodologies for the low-level determination of pyrethroids in water generally require significant sample preconcentration. This also can result in concentration of matrix components, which can adversely affect quantitation of the target compounds. The use of sensitive MRM-based methods has proved highly successful, and good sensitivity can be achieved in EI mode. However, pyrethroids ionize well in negative CI mode (NCI), and the selectivity of NCI is greater than EI for the pyrethroids because fewer compounds are ionized in this manner. Therefore, the matrix interference/background noise is reduced greatly, which allows very low LODs to be achieved (Figure 9). A further example of another pyrethroid, deltamethrin, determined in a real wastewater matrix at 0.007 mg/L, is shown in Figure 10.

Figure 10
Two MRM transitions were chosen so that quantification and confirmation could be performed in a single run. The confirmation criteria were based upon the measured ion ratio of the transitions with a tolerance of ±10% set. The expected ion ratio was calculated from the mean of the 10 calibration standards. For deltamethrin, the expected ion ratio was 0.987. The presence of deltamethrin was considered confirmed if the observed ion ratio from any extract did not deviate by more than 10% from this expected value. The ion ratio between the quantification transition (4546) and the confirmation transition (4412) for deltamethrin in Figure 10 is 0.971. The confirmation criteria have been passed with a percentage difference of 1.6% between the expected and measured ratios. This is significantly less than the acceptable limits of ±10%.
In summary, GC–MS-MS in NCI mode using MRMs provides high selectivity to reduce any matrix interferences, high sensitivity to reach the low reporting levels required, and quantitative and confirmatory data in a single injection even for potentially dirty samples such as wastewater.
Geochemical Application: Biomarkers in Oils
Biomarkers are natural products that can be traced to a particular biological origin and have a wide variety of applications, for example in petroleum exploration. Specifically, biomarkers in oil can reveal parameters such as the age of the source rock and the environment of deposition (such as marine or terrigenous). One of the main classes of biomarkers is the tetracyclic steranes, which originate from naturally occurring sterols in animals and plants that undergo diagenesis in sediments to form sterenes and, ultimately, steranes.
GC–MS is the principal method used to detect and identify sterane isomers and other biological markers. Where the concentration of biomarkers of interest is relatively high, analysis of characteristic fragment ions in single ion monitoring (SIM) mode (for example, using a single-quadrupole instrument) provides useful results.
However, some oils have very low concentrations of the biomarkers of interest and abundant interfering compounds. This type of analysis is prone to interferences from other families of compounds found in rock oil extracts, including hopanes, methyl steranes, and bicadinanes. In this case, tandem-quadrupole instruments (GC–MS-MS) in multiple reaction monitoring (MRM) mode provide a significant advantage over single quadrupoles, offering greater selectivity and sensitivity.

Figure 11
Figures 11 and 12 show both the SIM data and MRM data obtained from the analysis of a rock oil sample. These figures illustrate how the use of MRM can both overcome the interferences that limit the SIM experiments and provide additional highly specific structural information about the type of steranes present in the sample. Subtle differences in sterane hydrocarbon backbone are associated with specific organisms, and hence, the environmental origin of the oil (7,8).

Figure 12
Conclusions
Tandem-quadrupole mass spectrometers increasingly are recognized as the benchmark for accurate and reproducible quantitative analysis, providing exceptional sensitivity, selectivity, stability, and linearity even in the presence of complex sample matrices. They are powerful and flexible instruments extending the basic scanning and SIM capabilities of single quadrupole systems by offering the widest range of MS-MS experimental modes. Tandem-quadrupole GC–MS-MS is now widely regarded as a cost-effective, easy-to-use, benchtop solution, providing laboratories with access to the highest levels of performance that current and future applications will demand.
Acknowledgments
The authors gratefully acknowledge the following for collaborations resulting in the data presented in this article: Central Science Laboratory (York, UK); TNO (Zeist, Netherlands); CVUA (Stuttgart, Germany); Laberca, Ecole Nationale Vétérinaire (Nantes, France); RIKILT Institute of Food Safety (Wageningen, Netherlands); ALcontrol Laboratories (Rotherham, UK); Geological Survey of Denmark and Greenland (GEUS, Denmark).
Tim J. Jenkins and Gary Harland are with Waters Corporation, Manchester, UK.
References
(1) E. de Hoffman, J. Mass Spectrom., 31, 129–137, (1996).
(2) K.L. Busch, G.L. Glish, and S.A. McLuckey, Mass Spectrometry/Mass Spectrometry: Techniques and Applications of Tandem (John Wiley & Sons, New York, 1989).
(3) E. de Hoffmann, V. Stroobant, Mass Spectrometry: Principles and Applications (2nd Ed, John Wiley & Sons, Ney York, 2001).
(4) R.A. Yost, C.G. Enke, J. Am. Chem. Soc., 100, 2274 (1978).
(5) See for example www.faostat.fao.org for a rich source of trade statistics
(6) www.beyondpesticides.org/pesticides/factsheets/SyntheticPyrethroids.pdf
(7) K.E. Peters and J.M. Moldovan, Interpreting Molecular Fossils in Petroleum and Ancient Sediments (Prentice-Hall Inc, Englewood Cliffs, New Jersey, 1993).
(8) D.W. Waples and Tsutamu Machchara, Biomarkers for Geologists — A Practical Guide to the Application of Steranes and Triterpanes in Petroleum Geology, AAPG Methods and Applications Series No. 9 (American Association of Petroleum Geologists, Tulsa, Oklahoma, 1991).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.