Publication
Article
Spectroscopy
Inorganics II: The Spectra
Having defined inorganics and discussed the characteristics of their infrared spectra, we will begin a survey of the spectra of different inorganic functional groups. Included will be sulfates, carbonates, nitrates, silicates, and phosphates. Special attention will be paid to the stretching and bending vibrations of the polyatomic anions in these compounds.
Having defined inorganics and discussed the characteristics of their infrared spectra in the previous column (1), we will now start a survey of the spectra of different inorganic functional groups. Included here will be an introduction to the spectroscopy of inorganic polyatomic anions, and a discussion of the spectra of inorganic sulfates and carbonates.
From Last Time
The inorganics we will be studying consist of a positively charged ion, called the cation, and a negatively charged ion, called the anion. For the compounds we will be looking at, the cation will be a positively charged metal ion such as Na+, K+, or Ca+2, and the anions will be polyatomic, that is, consisting of more than one atom.
To review from last time (1), the characteristics of the infrared spectra of inorganics are (1–3):
- The majority of peaks are at low wavenumber due to the high atomic weight of the metal atoms found in inorganics. Many inorganic peaks fall in the far infrared, below the 400 cm-1 cutoff of most infrared spectrometers, and are thus not routinely observed.
- Most of the peaks from inorganics that appear in the mid-infrared (4000–400 cm-1) are from the stretching and bending vibrations of polyatomic anions. More on these below.
- The mid-infrared peaks of polyatomic anions are generally intense because inorganic bonds are frequently ionic, have large dipole moments, large values of (dµ/dx)2 (change in dipole moment with respect to bond length during a vibration [1]), and hence, strong fundamental infrared absorbances.
- Because the fundamental vibrations of inorganics give inherently intense peaks, overtone and combination bands frequently show up as well (1), sometimes with considerable intensity.
- A defining characteristic of inorganic spectra is the lack of C-H stretching peaks in the 3000 cm-1 vicinity.
- Water peaks are commonly found in the spectra of inorganics. These water molecules are found in three forms, adsorbed water, waters of hydration, and covalently bonded water (1).
- The same inorganic molecule can precipitate in different crystalline forms, which will have different infrared spectra (organics do this, too, which may be a topic for a future column).
The World of Polyatomic Anions
There are thousands upon thousands of inorganic molecules known to man. For our purposes, we will look at the spectra of the most common inorganic functional groups. As mentioned earlier, the most useful mid-infrared features of these functional groups are the stretching and bending peaks of polyatomic anions. As it turns out, the most common inorganic polyatomic anions all consist of non-metal atoms bonded to oxygens. For example, the inorganic carbonate anion, CO3-2, consists of an atom of the non-metal carbon bonded to three oxygens. The inorganic carbonate anion has an organic chemical analog, organic carbonates, or the CO3 group, whose spectra we have already studied (4); hence, the need to say, “organic carbonates” or “inorganic carbonates.” The structure of both types of carbonate are shown in Figure 1.
FIGURE 1: (a) The chemical structure of an organic carbonate, CO3. (b) The chemical structure of an inorganic carbonate anion, CO3-2.
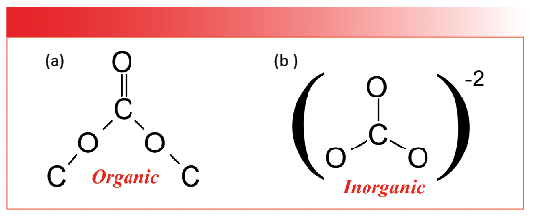
Note that nominally organic and inorganic carbonates have the same chemical formula, CO3. However, the chemical bonding in these two carbonates is completely different. Organic carbonates contain covalent carbon-oxygen bonds, and, as shown in Figure 1, note that organic carbonates contain one C=O bond and four C-O bonds. As we saw (4), the C=O stretch of organic carbonates fall around 1750 cm-1 (going forward all peak positions will be in cm-1 units), whereas the midpoint for where C-O stretching peaks fall is about 1150. Note, however, that inorganic carbonates do not contain individual C=O or C-O bonds, all three carbon-oxygen bonds are equivalent, and their bond order falls somewhere between one and two. This is going to be typical of the polyatomic anions we study—the nonmetal-oxygen bonds in these anions will have the same bond order and may not fit into the simple single bond/double bond picture that describes so many organics.
We can prove to ourselves that the bond order in inorganic carbonates is somewhere between one and two. We already know that the stretching peaks from C=O bonds in organic carbonates fall at 1750, and that C-O stretching peaks tend to fall at 1150. If we average these two quantities, we get
(1750 cm-1 + 1150 cm-1)/2 = 1450 cm-1
Thus, we can predict that the carbon-oxygen bonds for inorganic carbonates with a bond order of 1.5 will have stretching peaks around 1450. This is not far off the mark, as seen in the spectrum of calcium carbonate shown in Figure 2 (available by accessing the QR code at the end of the article).
FIGURE 2: The mid-infrared spectrum of calcium carbonate, CaCO3. Note the carbon-oxygen stretching peak at 1463 and carbon-oxygen bending peak at 877.
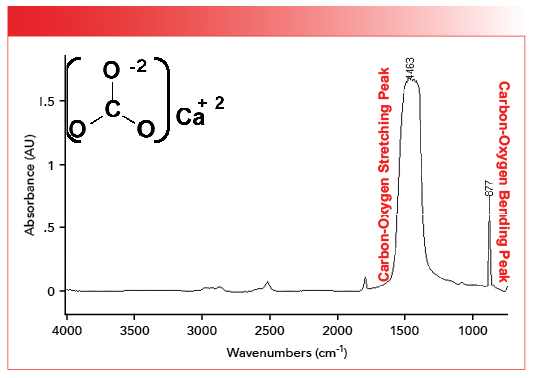
The peak at 1463 in the spectrum of calcium carbonate falls not far from our predicted value of 1450. This peak means that the carbon-oxygen bond order in inorganic carbonates is close to 1.5. The fact that there is only one carbon-oxygen stretching peak indicates the carbon-oxygen bonds all have equivalent bond orders. We will see this in the spectra of the polyatomic anions we will be studying in this and the next column.
Inorganic Sulfates
Like the carbonates discussed above, sulfates come in organic and inorganic forms. However, we have not yet discussed organic sulfates, which will be taken care of in a later column. The structures of the two types of sulfates are shown in Figure 3.
FIGURE 3: The chemical structures of (a) organic and (b) inorganic sulfates.
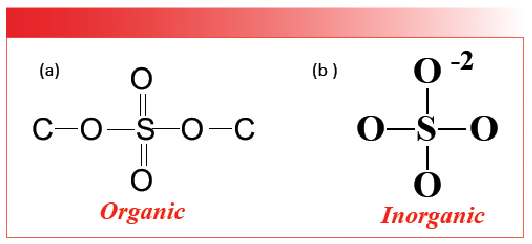
Note that both structures contain a SO4 moiety. However, in organic sulfates, the bonds are covalent with S-O and S=O bonds, whereas, in inorganic sulfates, the bonds are ionic and the bond orders of all four sulfur-oxygen bonds are equivalent. We can see this by doing a calculation for sulfates similar to the one we did for carbonates above. In organic molecules, S=O stretching peaks fall around 1200 and S-O stretching peaks are found in the vicinity of 1000 (2). We can then calculate that the stretching peak for a sulfur-oxygen bond and half might fall at the average of these two numbers which is 1100. The spectrum of gypsum, calcium sulfate dihydrate, is seen in Figure 4 (available by accessing the QR code at the end of the article).
FIGURE 4: The infrared spectrum of the inorganic compound calcium sulfate dihydrate (gypsum), CaSO4•2H2O. Note the sulfur-oxygen stretching peak at 1133.
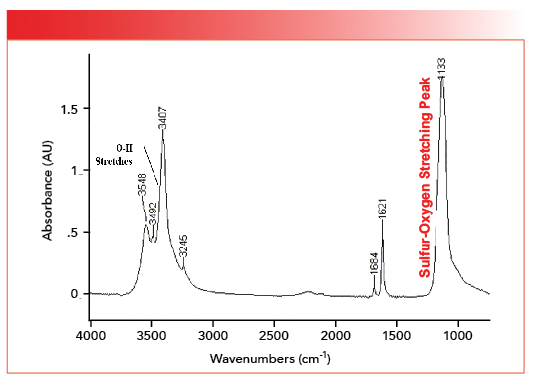
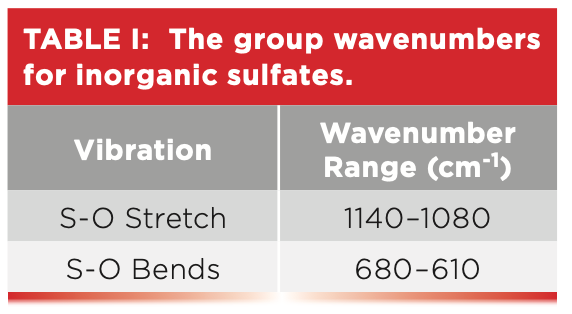
The sulfur-oxygen stretching peak in Figure 4 falls at 1133, not far off from our prediction. This peak position indicates that the sulfur-oxygen bond order in inorganic sulfates is on the order of a bond and a half. For inorganic sulfates, generally, sulfur oxygen stretching peaks fall between 1140 and 1080. The existence of only one sulfur-oxygen stretching peak, rather than individual S=O and S-O stretching peaks as found in covalently bonded sulfur compounds, indicates the bond order of all the sulfur-oxygen bonds in inorganic sulfates is equivalent. Note that the spectrum in Figure 4 cuts off at about 750 because it was measured with an infrared microscope with a highly sensitive mercury cadmium telluride (MCT) detector (5), which, unfortunately, does not go down to 400. The stretching peaks from the waters of hydration in Figure 4 are noted, and were discussed last time (1). The spectrum of another inorganic sulfate, sodium sulfate (Na2SO4), is seen in Figure 5 (available by accessing the QR code at the end of the article).
FIGURE 5: The infrared spectrum of sodium sulfate. The sulfur-oxygen stretching peak is at 1135, and the sulfur oxygen bending peaks are at 639 and 617. Note the overtone and combination bands, and the peak from the O-H stretch of adsorbed water at 3454.
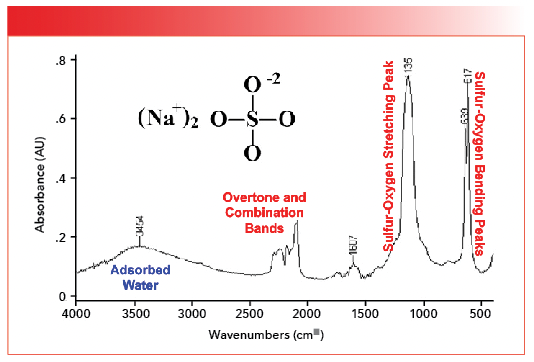
The adsorbed water, overtone, and combination band peaks were discussed previously (1). The single sulfur-oxygen stretching peak is seen at 1135, only two wavenumbers different than the same peak in the spectrum of gypsum seen in Figure 4.
Like most of the other functional groups we have discussed in this column series, polyatomic anions have stretching and bending vibrations. As we will see, the number and position of polyatomic anion bending peaks will be useful in distinguishing different inorganics from each other. In Figure 5, the sulfur-oxygen bending peaks are found at 639 and 617, and, in general, both peaks from 680 to 610. We didn’t see these peaks in Figure 4 because the spectrum cut off at 750. Table I summarizes the group wavenumbers for inorganic sulfates.
Note in Figures 4 and 5 that the sulfur-oxygen stretching peaks are broadened, whereas in Figure 5 the sulfur-oxygen bending peaks are quite narrow and sharp. This is typical of the spectra of inorganic polyatomic anions as we will see going forward. The sharpness of polyatomic anion bending peaks makes them better suited for quantitation than broadened sulfur-oxygen stretching peaks (6).
Inorganic Carbonates
The spectrum of an inorganic carbonate, calcium carbonate, is seen above in Figure 2. In our daily lives, calcium carbonate is otherwise known as chalk and limestone. As we discussed last time (1), like many inorganics, calcium carbonate is found in different crystalline forms which give different infrared spectra. The spectrum in Figure 2 is of the aragonite form of calcium carbonate. Also recall from above that the carbon-oxygen bond order in inorganic carbonates is about 1.5, and that all the carbon-oxygen bonds are equivalent.
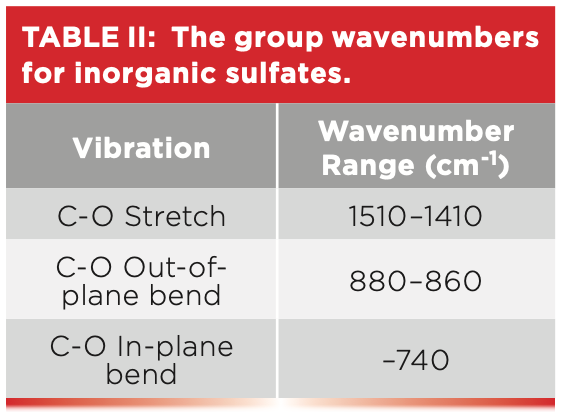
The carbon-oxygen stretching peak in Figure 2 is seen at 1463, and, for inorganic carbonates, generally this peak falls from 1510 to 1410. Note that this peak is broad and intense like the sulfur-oxygen stretching peaks seen in Figures 4 and 5, but it falls at a higher wavenumber. There are two bending peaks for inorganic carbonates: the in-plane bend falls from 880 to 860, and the out-of-plane bend is found around 740. In Figure 2, the in-plane bending peak is seen at 877, and the out-of-plane bending peak is not seen, because, unfortunately, the spectrum cuts off at 750, right before where this peak would appear. Note the 877 peak in Figure 2 is nice and sharp like the other polyatomic anion peaks seen in Figure 5. Table II shows the group wavenumbers for the inorganic carbonate functional group.
Conclusions
We reviewed the unique aspects of inorganic infrared spectra. We then discussed inorganic polyatomic anions and how they differ from their organic analogs; specifically the bonds in polyatomic anions all have the same bond order, and often have a bond order between 1 and 2. We wrapped up with a discussion of the infrared spectra of sulfates and carbonates. We found their stretching vibration peaks to be broad and intense, and their bending vibrations peaks to be narrow and sharp—making them excellent for quantitative work.
References
(1) Smith, B. C., Inorganics I: Introduction. Spectroscopy 2023, 38 (11), 18–21. DOI: 10.56530/spectroscopy.rp3780c4
(2) Smith, B. C. Infrared Spectral Interpretation: A Systematic Approach, CRC Press, Boca Raton, 1999.
(3) Nyquist, R.; Kagel, R., Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts, Elsevier, 1971.
(4) Smith, B. C., The C=O Bond, Part VII: Aromatic Esters, Organic Carbonates, and More of the Rule of Three. Spectroscopy 2018, 33 (9), 24–28.
(5) Smith, B. C., Fundamentals of Fourier Transform Infrared Spectroscopy 2nd Ed.; CRC Press, 2011.
(6) Smith, B. C. Quantitative Spectroscopy: Theory and Practice; Elsevier, 2002.
Brian C. Smith, PhD, is the founder and CEO of Big Sur Scientific, a maker of portable mid-infrared cannabis analyzers. He has over 30 years experience as an industrial infrared spectroscopist, has published numerous peer-reviewed papers, and has written three books on spectroscopy. As a trainer, he has helped thousands of people around the world improve their infrared analyses. In addition to writing for Spectroscopy, Dr. Smith writes a regular column for its sister publication Cannabis Science and Technology and sits on its editorial board. He earned his PhD in physical chemistry from Dartmouth College. He can be reached at: SpectroscopyEdit@MMHGroup.com ●


Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.




