Measuring Cellular-Scale Nutrient Distribution in Algal Biofilms with Synchrotron Confocal Infrared Microspectroscopy
The authors use synchrotron confocal IMS to measure the response of two dominant algal species from a stream of biofilm to a reduction in nutrient availability.
The microscope and infrared spectrometer are two of the most useful tools for the study of biological materials, and their combined analytical power far exceeds the sum of the two. Performing molecular spectroscopy through a microscope superimposes chemical information onto the physical microstructure obtained from the optical microscope when visible and infrared information are collected under the same conditions. The instrument developments that enable current infrared microspectroscopic studies began with the introduction of the first research-grade infrared microscope, patented in 1989 (1). By 1993, published reports using this method to determine macroalgae (seaweed) cell-wall composition appeared (2-4). Since these initial reports, the use of infrared microspectroscopy (IMS) in microalgal (single cells or groups of cells) research has grown. Primarily, cultured algae have been used to hone IMS methodology and evaluate its capabilities in algal research (5–8). Studies involving natural, mixed species assemblages, which can utilize the spatial resolution potential of this technique fully are rare (9–11). For instance, in a recent review of IMS microalgal ecological research (12), only 3 of the 29 peer-reviewed publications investigated natural algal assemblages. Both thermal and synchrotron infrared sources provide a resolution capable of measuring individual algae in mixed species assemblages, and each has its advantages. For example, thermal source IMS is more accessible, allowing more samples to be analyzed than synchrotron IMS. However, synchrotron IMS with confocal masking provides superior resolution, which can be critical in isolating small or contiguous cells.
Algal ecology is the study of the interaction between algae and their environment. Infrared microspectroscopy addresses a major logistical problem in this field, obtaining species-specific cellular biochemical information from natural, mixed-species assemblages (11,12). Benthic (bottom-dwelling) algae, for example, grow in a three-dimensional matrix (biofilm) composed of different cell sizes, shapes, and configurations. The optical and ecological challenge of studying algae is apparent from Figure 1, which shows a photomicrograph of algal chlorophyll fluorescence on a rock. Several issues make it difficult to obtain single species measurements with standard techniques: cell sizes can vary over an order of magnitude; species can occur as single cells, long filaments, or globular colonies; a number of different species can be found within a few square millimeters; and fluorescence can vary across cells (that is, the physiological state varies across cells).
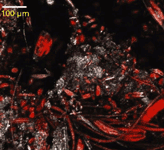
Figure 1: Micrograph of algal chlorophyll fluorescence (red) on a rock (white) collected with confocal laser scanning microscopy. Note the variety of cell shape configurations and sizes among the benthic algal species. Larger species and colonies are routinely analyzed by conventional IMS. The small size of many species (

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.