Article
Spectroscopy
Spectroscopy
Observing Heterogeneous Catalysts While They Are Working: Operando Raman Spectroscopy
The opportunities and current progress of operando Raman spectroscopy are presented through examples based on the authors' work.
Operando means working, thus, this technique refers to the combination of a characterization study of the surface of a catalyst at the same time that the activity is being monitored. This approach, which permits the simultaneous characterization of both parameters in a single experiment, facilitates uncovering the relationships between structure and activity. Such information is critical to improving the performance and formulation of catalysts. We illustrate the possibilities of operando Raman spectroscopy with four examples.
The synthesis of most chemicals requires a catalytic process. As a result, catalysts are often studied with the goal of improving their properties and catalytic behavior. Spectroscopy is a valuable tool to characterize the surface of solid (heterogeneous) catalysts and also can be useful for the determination of reaction intermediates and other adsorbed species. Raman spectroscopy is particularly useful for studying catalysts because Raman spectra can be obtained under a wide range of conditions (at high pressures and at temperatures above 1000 °C, by the use of the appropriate excitation wavelength), which makes it possible to use Raman spectroscopy to characterize catalysts during a reaction. Determining catalyst performance (such as conversion or selectivity) during a reaction, at the same time that the catalyst is characterized, is the concept of the operando Raman methodology (1–6).
Over the last decade, operando Raman spectroscopy has been applied successfully to the study of several catalytic systems, in both liquid- and gas-phase processes. Such studies have provided a better understanding, and subsequent improvement, of various catalytic processes. The aim of this article is not to provide a detailed review of the method, but rather to present briefly the opportunities and current progress of operando Raman spectroscopy through some examples based on the authors' work.
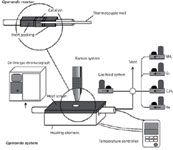
Figure 1: An operando Ramanâgas chromatography setup. Adapted from reference 9 (with permission).
Look, But . . . How?
One of the critical issues in performing operando experiments is to correctly design the Raman cell, because it has to perform like a catalytic reactor and meet the optical requirements for spectroscopy. The Raman cell and the reaction conditions must prevent any mass or heat transfer limitations and the contribution of noncatalytic reactions such as gas-phase reactions. Thus, conventional cells cannot be used to perform catalytic tests. Traditional cells are good for in situ studies, because the sample is fully "aware" of the presence of the gas, but most in situ Raman cells are designed in such a way that not all the gas flowing through it interacts with the sample (catalyst). Figure 1 shows the homemade operando reactor designed by our group that was used to perform the operando experiments discussed in this article. Essentially, it is a fixed-bed catalytic reactor with walls that are optically appropriate for Raman spectroscopy. To prevent the participation of homogeneous reactions, the reactor was designed to minimize gas-phase activation of reactants (by not having any void volume). Thus, the operando reactor makes it possible to obtain Raman spectra of catalysts and genuine catalytic data. Figure 2 shows the results obtained for several catalysts at different temperatures in the operando reactor and also in a conventional fixed-bed catalytic reactor; the activity and selectivity obtained in both reactors is essentially the same, thus proving the validity of the catalytic tests performed during the operando Raman experiments in this homemade reactor cell.

Figure 2: Propane conversion vs. temperature and selectivity to acrylic acid for several catalysts obtained in a conventional fixed-bed reactor and in an operando reactor. Propane partial oxidation conditions: C3H8/O2/H2O/He = 12.5/20.4/15.9/51.2; 4800 h-1; 0.2 g of catalyst. Adapted from reference 13 (with permission).
When the Active Phases Are Sensitive to the Environment
There are many cases in which the active phases of a catalyst form, or, at least change, during reaction. Such phases may not be present before or after catalysis, but only during catalysis. The following examples illustrate two cases, one in which the reaction conditions shape the working catalyst structure, and one in which we monitored the preparation of the active phase.

Figure 3: Raman spectra of SbâVâO/Al2O3 catalyst during a propane ammoxidation reaction: (a) dehydration at 200 °C; (b) ammoxidation at 200 °C; (c) 400 °C; (d) 420 °C; (e) 480 °C; (f) reoxidation at 440 °C. The corresponding yield values are presented in the left panel. Reaction conditions: 200 mg of catalyst, total flow 20 mL/min; feed composition (% volume); C3H8/O2/NH3/H2O/He (9.8/2â .6/56.5). Adapted from reference 7 (with permission).
Case I: VSbO4 Active Phase During the Propane Ammoxidation Reaction
Activating alkanes to obtain commodity chemicals is one of the major challenges the chemical industry must solve in the coming years. The interaction between surface antimony oxide, with very weak Raman bands, and surface vanadium oxide species, with Raman bands at 1020 and 900 cm-1 (Figure 3, spectrum of dehydrated sample), takes place under reducing or non net-oxidizing environments (such as ammoxidation reaction conditions) leading to the formation of the trirutile VSbO4 phase, characterized by a broad Raman band at 840 cm-1 and segregated α-Sb2O4, as shown by the spectra taken during ammoxidation (Figure 3). The catalyst is selective to acrylonitrile (yield close to 30%) when the trirutile band is intense, indicative that VSbO4 is present on the surface of the catalysts. The interaction between Sb and V is partially reversed upon reoxidation, where reduced vanadium oxide species segregate as surface V5+ species, characterized by a band near 1024 cm-1 (Figure 1, reoxidized sample). Thus, operando Raman spectroscopy uncovers the redox cycle taking place on the VSbO4 active phase during propane ammoxidation (Figure 4) (7–9).
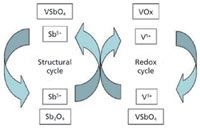
Figure 4: Possible catalytic redox cycle of vanadium and migration cycle of antimony during propane ammoxidation in V-Sb-O catalysts. Adapted from reference 9 (with permission).
Case II: Ni-Mo Hydrotreatment Catalysts
Ni-Mo–based catalysts are promising for hydrodesulfurization reactions, which are important processes in the oil refining industry. Hydrodesulfurization eliminates sulfur and other contaminants from fossil fuels and intermediate petroleum distillates. Figure 5 shows representative Raman spectra obtained during sulfidation of Ni-Mo–oxide based catalysts (10). At the initial sulfidation stages, below 300 °C, oxysulfides and less-reduced molybdenum sulfide phases (MoS3) are apparent. The lack of Raman features at 300 °C during the sulfidation process is probably a result of a very broad distribution of states, because Raman bands of nanocrystalline MoS2 become apparent at 400 °C. Thus, the spectra show that there is a partial sulfidation process at temperatures up to 300 °C, and that an important rearrangement happens before the formation of an incipient and defective MoS2 structure that is beneficial to hydrodesulfurization activity.

Figure 5: In situ Raman spectra at different sulfidation stages of NiMoâ50ASA. Adapted from reference 10 (with permission).
Understanding Deactivation Processes
Given that reactions may lead to progressive deactivation of catalysts, the simultaneous determination of structure and activity offers the opportunity to understand the deactivation phenomena and infer the nature of the active site. The examples presented below illustrate this possibility.
Case III: Ce-V-O–Based Catalysts During Ethane Oxidative Dehydrogenation
Understanding the mechanism and active phases of Ce-V-O–based catalysts is very important, because both vanadia and ceria are used for many catalytic applications. It has been reported that ceria-supported vanadia catalysts form CeVO4 at lower temperatures than upon calcination in air (11). This is because of the redox cycle during the reaction. The redox cycle periodically reduces ceria sites, promoting the solid-state reaction to form CeVO4 (an irreversible reaction), with the subsequent deactivation of the catalysts. Martinez-Huerta and colleagues (11) monitored the transformation of surface vanadium oxide species on ceria into CeVO4 during an ethane oxidative dehydrogenation reaction. That study detected how the catalysts deactivated at reaction temperatures above 500 °C; the operando Raman–gas chromatography (GC) study shows an incipient formation of CeVO4 on the surface of catalysts with no appreciable deactivation at 460 °C. The operando study (Figure 6) shows that the Raman bands of CeVO4 become sharper during reactions at temperatures greater than 500 °C. This trend is consistent with a decrease in the exposure of the active sites rather than with a change in the structure of the active phase. The Arrhenius plots of activity data measured in the operando Raman cell (Figure 6) show that the apparent activation energy does not change significantly as the solid-state reaction transforms ceria-supported vanadium oxide species into CeVO4. The Arrhenius plots underline a decrease in the number of active sites but not a change in the nature of the active sites. These data lead to the conclusion that V-O-Ce bonds present in both the fresh (ceria-supported vanadia) and aged (CeVO4) catalysts are directly related to the active sites, and that the redox cycle is related to the cerium ions at the interface with vanadia. Figure 6 (right) illustrates how the progressive interaction between ceria support and surface vanadium oxide species stabilizes Ce3+ ions at the vanadia–ceria interface. After sharing these data, Sauer's and Freund's groups performed a detailed experimental and density functional theory (DFT) study of this interaction that showed that the preferred interface between dispersed vanadia and ceria support involves reduced Ce3+ ions in the V-O-Ce bonds and confirmed that the preferred oxidation state for vanadium is V5+, which is nearly impossible to reduce (12).

Figure 6: Left: Arrhenius plot of ethane conversion vs. reaction temperature in the operando fixed-bed reaction cell. Right: Qualitative illustration of dynamic states of the V5 +/CeO2 system during the incipient and extensive formation of CeVO4. Adapted from reference 11 (with permission).
Case IV: Multioxide Mo-V-Nb-Te-O Catalysts for the Partial Oxidation of Propane into Acrylic Acid
The selective oxidation of propane into acrylic acid is an interesting route for alkane valorization. The Mo-V-Nb-Te-O catalytic system is active in partial oxidation reactions of propane. Tellurium is a critical component, the role of which recently has been uncovered using operando Raman–GC (13). Operando Raman–GC shows that the shape of the spectra changes drastically when the temperature reaction is increased to 375 °C (Figure 7). Below that temperature, Raman bands near 815 and 380 cm-1 assigned to Mo–V–O structures and bands near 1000 cm-1 assigned to MoOx or VOx dominate the spectra. In addition, Raman bands near 760 and 230 cm-1, assigned to AlVMoO7 structures, can be detected along with the Raman band near 880 cm-1, assigned to an Mo5O14-type structure. Conversely, Raman bands near 960, 780, and 237 cm-1 dominate the spectra when the reaction temperature reaches 375 °C; such Raman bands are indicative of a distorted MoO3 oxide with some minor amounts of vanadium species (14). The amount of such an MoO3 structure is not very large, because no X-ray diffraction (XRD) pattern for a similar structure was detected, but its bands dominate the Raman spectra because this phase possesses a very high Raman cross-section and Raman spectra uncover important changes at the nanometer scale during the reaction. In addition to monitoring changes occurring in the Nb–Mo–V–O structures during propane oxidation, operando Raman–GC experiments show the formation of coke because bands appearing in the 1200–1650 cm-1 region are attributed to carbon deposits with sp2 hybridization.

Figure 7: Operando RamanâGC spectra during the selective oxidation of propane on 12Mo5V4N±; Left: Raman spectra obtained during reaction at the temperature indicated; right: simultaneous activity and selectivity data obtained during Raman spectra acquisition. C3H8/O2/NH3/H2O/He = 12.5/20.4/15.9/51.2; 4800 h-1; 0.2 g of catalyst. Adapted from reference 13 (with permission).
A similar operando Raman–GC experiment was performed with a catalyst doped with tellurium. As Figure 8 shows, the addition of a small amount of Te to the Mo-V-Nb oxide system inhibits the deactivation of the catalyst by its rearrangement into distorted MoO3 structures and the buildup of carbonaceous deposits. In this case, Raman bands in the 900–1020 cm-1 range and the Raman bands near 820 and 370 cm-1 of dispersed oxides are apparent during a reaction at 350 °C. Such dispersed oxide structures blend into distorted rutile structure phases as the reaction temperature increases (to 375–450 °C) and are characterized by a broad Raman signal near 800 cm-1. Thus, the study demonstrated that tellurium doping generates highly distorted rutile structures during the reaction that are capable of inhibiting the formation of MoO3 crystallites under reaction conditions and improves the performance of these catalysts to achieve sufficient performance.

Figure 8: Operando RamanâGC spectra obtained during the selective oxidation of propane on 12Mo5V4Nb0.5Te0.5. Left: Raman spectra during the reaction at the temperature indicated. Right: Simultaneous activity and selectivity data obtained during Raman spectra acquisition. Reaction conditions: C3H8/O2/H2O/He = 12.5/20.4/15.9/51.2; 4800 h-1; 0.2 g of catalyst. Adapted from reference 13 (with permission).
Conclusions
The operando Raman methodology combines structural and catalytic measurements in a single experiment. Because the molecular structure of a catalyst depends on its specific environmental conditions, this combination is critical to be able to reliably assess structure–activity relationships at the molecular level. The examples presented here illustrate that this methodology can follow the dynamic states of catalysts, which are determined by the reaction. The formation of active phases during the reaction and their eventual deactivation are directly linked to kinetic data. In addition, it has been shown that Raman spectroscopy uncovers important changes at the nanometer scale during reactions, even though those changes are not revealed by XRD. In summary, the state of a catalyst is determined by the reaction conditions. This could be expressed borrowing the expression by the Spanish philosopher Ortega y Gasset, "I am I plus my circumstances." The examples presented here tell us that the catalyst is itself and its circumstances.
References
(1) B.M. Weckhuysen, Chem. Commun. 97–110 (2002).
(2) M.A. Bañares, M.O. Guerrero-Pérez, J.L.G. Fierro, and G.G. Cortéz, J. Mater. Chem. 12(11) 3337–3342 (2002).
(3) M.O. Guerrero-Pérez and M.A. Bañares, Catal. Today 113, 48–57 (2006).
(4) V. Calvino-Casilda and M.A. Bañares, Catalysis 24, 1–47 (2012).
(5) M.A. Bañares, Adv. Mater. 23, 5293 (2011).
(6) I.E. Wachs and C.A. Roberts, Chem. Soc. Rev. 39, 5002 (2010).
(7) M.O. Guerrero-Pérez and M. A. Bañares, Chem. Commun. 1292–1239 (2002).
(8) M.O. Guerrero-Pérez and M. A. Bañares, J. Phys. Chem. C 111, 1315 (2007).
(9) M.O. Guerrero-Pérez and M. A. Bañares, Catal. Today 96, 265 (2004).
(10) M.O. Guerrero-Pérez, E. Rojas, A. Gutiérrez-Alejandre, J. Ramírez, F. Sánchez-Minero, C. Fernández Vargas, and M.A. Bañares, Phys. Chem. Chem. Phys. 13, 9260 (2011).
(11) M.V. Martínez-Huerta, G. Deo, J.L.G. Fierro, and M.A. Bañares, J. Phys. Chem. C 112, 11441 (2008).
(12) M. Baron, H. Abbott, O. Bondarchuk, D. Stacchiola, A. Uh, S. Shaikhutdinov, H.-J. Freund, C. Popa, M.V. Ganduglia-Pirovano, and J. Sauer, Angew. Chem. Int. Ed. 48, 8006 (2009).
(13) R. López-Medina, J.L.G. Fierro, M.O. Guerrero-Pérez, and M.A. Bañares, Appl. Catal. A 406, 34 (2011).
(14) M.A. Bañares and S.J. Khatib, Catal. Today 96, 251 (2004).
M. Olga Guerrero-Pérez is with the Department of Chemical Engineering at the University of Malaga in Malaga, Spain. Please direct correspondence to: oguerrero@uma.es.
M.A. Bañares is with the Catalysis and Petrochemical Institute of the Consejo Superior de Investigaciones Científicas (CSIC), in Madrid, Spain.

Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.



