Our Daily Dose of Poison: A Look at Lead in the Food Supply
Spectroscopy
How much lead is in our daily lives? We take a look at current research concerning lead in the United States food supply and investigations using ICP-MS into the measurement of high concentrations of lead in food.
This month’s “Atomic Perspectives” examines current research concerning lead in the United States food supply as well as investigations using inductively coupled plasma–mass spectrometry (ICP-MS) carried out by the author into the measurement of high concentrations of lead in food items, which in many cases far exceeded the levels found in the drinking water supply in Flint, Michigan. The study also briefly covers some history of lead use and the challenges and limitations of setting advisory limits and guidelines.
Lead is one of the most documented and ubiquitous toxic substances in the world present in soil, plants, water, and air. Industrial activities and lead products transitioned a mostly immobile element into a highly dispersed toxic pollutant. In 2016, people in the United States were shocked over the high lead levels detected in the drinking water supply in Flint, Michigan. The analytical testing community was concerned, but not truly surprised. The bigger surprise for scientists was the public reaction to this one incident rather than the thousands of other cases of lead exposure the scientific community uncovers each year. Over the centuries, lead has been dispersed by daily use of lead products, factory emissions, gasoline combustion, paint decay, pesticide application, and other industrial uses.
Historical Perspective
The earliest instances of the use of lead came from Asia Minor where small lead beads were discovered dating back to about 6500 BCE. The early Egyptians used lead to glaze their earthenware and applied lead cosmetics. Recently, studies have suggested they mixed multiple forms of lead such as galena, cerussite, laurionite, and phosgenite in the cosmetic, kohl, to ward off illnesses. The antibacterial properties of these compounds may have played a role in that belief (1).
Ancient Greeks were experts in the process of converting lead ore to white lead (basic lead carbonate), which became the base pigment for paints, coatings, and cosmetics for centuries. Lead was an appealing metal to work with because of its low melting point and ability to bond easily with other elements. It was resistant to corrosion and inexpensive to obtain because it was a common by-product of gold and silver mining.
The Romans expanded lead use to between 60,000 to 80,000 tons of lead per year at peak production. Romans used lead in all of their daily activities in applications ranging from water pipes to tableware and cosmetics. Lead "sugar" (lead [II] acetate) was a popular additive to food and drinks to sweeten possibly sour or spoiled food. Another popular sweetener, sapa, was grape juice syrup reduced in lead pots to produce the sweet taste not found by using copper or brass cookware. It is suggested that the average Roman's daily exposure from all sources of lead was between 35 and 250 mg/day (2).
Despite the widespread use of lead, there were indications that ancient physicians and scholars were aware of its potential toxicity. In 400 BCE, Hippocrates described symptoms and illnesses related to the consumption of lead-laden food and wine. In 250 BCE, the Greek poet and physician, Nikander of Colophon, reported cases of anemia, colic, and paralysis from lead poisoning. Throughout the Roman Empire, gout, thought to be caused by lead, plagued the affluent classes. In the ancient world, lead poisoning was a disease of either the wealthy who could afford luxuries such as lead-lined wine casks, pewter dinnerware, and lead-laden cosmetics or the slaves exposed to lead in the mines and refineries.
Industrial Uses
Lead use continued through the subsequent centuries, albeit at a decreased amount after the fall of the Roman Empire. The middle ages saw the use of lead to create glass, bullets, and cosmetics to create the pale, white appearance popularized by Queen Elizabeth I. As new worlds were discovered, the mining of lead expanded to North America with the first mine established in 1621 in Virginia. Lead production increased dramatically with the dawn of the Industrial Revolution. Great Britain's production surpassed the levels seen at the height of the Roman Empire's production. By 1900, the United States had surpassed the United Kingdom in lead production to meet the world's increasing demand, which had grown dramatically from Rome's peak production of 80,000 tons per year to over 11 million tons per year by 2016 (3).
Many of the modern sources of lead pollution are our inheritance and will be our legacy. During the industrial revolution through the beginning of the 20th century, the largest sources of lead were industrial emissions and lead products. This exposure distribution dramatically changed during the 1920s with the introduction of tetraethyl lead (TEL) into automotive gasoline. From that point onward, the largest sources of lead exposure became automotive emissions and lead paint. The addition of TEL to gasoline has been described as one of the greatest public health failures of the 20th century.
Leaded Gasoline and Paint
In the 1850s, TEL was discovered by a chemistry professor at the University of Zurich named Carl Jacob Löwig. During the 1920s, automakers saw stiff competition in the race to increase engine performance. A General Motors chemist, Thomas A. Midgley, found that the addition of TEL allowed engines to run without knocking, and a new product called ethyl gas was born. From the outset, many concerns were voiced by scientists familiar with the product. Letters were written objecting to the use of the toxic element. By the 1920s, the dangers of lead were well known. The first U.S. childhood death attributed to lead paint had been reported in 1914 and by the time of the launch of ethyl gas, the League of Nations had banned lead interior paint. By the mid-1930s and 1940s the use of leaded gasoline was firmly entrenched in the automotive industry.
Just a century after its discovery, TEL became a major pollutant in the world. Despite growing health questions, in 1959, the U.S. Public Health Service approved a request to increase lead levels in Ethyl Corp.'s gasoline. The 1960s saw the first investigations and hearings into the restriction of lead in gasoline, but it wasn't until 1980 that an official phase-out was enacted. In 1980, the National Academy of Sciences stated that leaded gasoline was the greatest source of lead pollution in the atmosphere and it was estimated that the daily intake was approximately 0.3 mg per person from this pollution.
Unfortunately, our inherited legacy of lead persists. Many buildings have decades if not centuries of lead paint or were constructed with lead plumbing. One of the primary routes of lead poisoning today in children is exposure through household and neighborhood dust and dirt that contains leaded automotive residues and deteriorating lead paint (4). Urban cities with high populations and dense traffic bear the highest lead burden. Studies in New Orleans, Louisiana, and Saint Paul, Minnesota, showed that the cities with higher automotive congestion could have anywhere between 100 to 1200 µg/g of lead in the soil, whereas the suburban areas were found to have less than 75 µg/g of lead in the soil on average (5).
Drinking Water
Another major route of lead ingestion for children is drinking water. Lead enters the drinking water in a variety of means, but one prevalent method is lead from plumbing. Water leaches the lead from old pipes and contaminates the water. A second route of lead entering the drinking water is the contamination of the ground water by residual lead sources (dust, dirt, paint, old pesticides, and so on). The 1974 Clean Water Act transferred governance over drinking water to the federal government. The United States Environmental Protection Agency (US EPA), acting for the federal government, has imposed restrictions for water quality. In 1991, the Lead and Copper Rule was imposed by the EPA to limit the concentration of lead and copper in public drinking water at the consumer's tap. The rule also issues guidelines regarding the amount of lead and copper that can be released through pipe corrosion. The action level for lead is 15 µg/L. If a public water supply exceeds the action limit, appropriate treatments must be used to reduce the lead levels to below the action limit.
In 2015 and 2016, the news was filled with the public health disaster that was being uncovered in Flint, Michigan (6). For decades, the city of Flint had obtained their water from the Detroit Water and Sewerage Department (DWSD), which pumped its water from Lake Huron. The DWSD, following the guidelines of the Lead and Copper Rule, treated the water with orthophosphates (often in the form of food-grade phosphoric acid), which coat the water pipes and reduce leaching of lead and copper into the water supply. While Flint was connected to this treated water supply, the lead and copper levels in their drinking water complied with the EPA rules.
Crisis in Flint, Michigan
In 2014, Flint's administration decided to switch the water supply from Lake Huron to the Flint River. To the local residents, the Flint River was not known as a trustworthy body of water. It had a reputation of being polluted. The water was much more corrosive than the water of Lake Huron. In addition to a lack of organophosphate treatments, the water of the Flint River was treated with ferric chloride to reduce the formation of trihalomethanes from organic matter, which dramatically increased the chloride content and made the water increasingly corrosive.
By April 2014, the city had switched its water supply to the Flint River and soon after, reports of dirty and smelly water started coming into city offices. By the summer of 2015, scientists were finding drinking water with high lead content. In the summer of 2016, scientists at Virginia Tech (VT) began studying lead levels in the Flint water and by the fall had found more than 40% of homes in Flint had high lead levels. In September, they recommended that the state of Michigan should declare the drinking water in Flint unsafe for consumption. The most significant measure of the contamination was the 90th percentile level of lead exposure in homes tested, meaning that 90% of homes would have a lead level below that threshold and 10% would have levels above it. The action level for drinking water is 15 µg/L, and in the Detroit water supply, which was previously connected to Flint, it was measured at 2.3 µg/L. At the time of the crisis, the 90th percentile of Flint's water supply was 27 µg/L with some samples reading over 100 µg/L. One extremely high sample was found to have 13,000 µg/L, which could be defined as toxic waste by the EPA, whose definition is 5000 µg/L (5 ppm) or higher (7) (see Table I).

CLICK TABLE TO ENLARGE
The public response to the crisis in Flint was one of shock and horror. Demands were made to improve clean water access and the government funds for water quality. The reaction of the scientific community was one of resignation and disappointment. Scientists had been speaking out about lead toxicity for more than a century, but often the reports of lead contamination or poisoning fell on deaf political ears while being quietly logged in the scientific journals. During the past two decades, more than one million articles concerning lead exposure have been published, which equates to almost 150 articles published every day for the past 20 years.
Flint was not the first major lead contamination event in the United States, it was just one of the most publicized. At the height of the Flint controversy, it was revealed by CNBC that the EPA found only nine U.S. states routinely reported safe lead levels in their water supply (8). A total of 41 states had action levels that exceeded the safe lead limit within the last three years. Reuters issued a report at the end of 2016 that more than 3000 areas in the United States had higher lead levels than Flint and some levels were twice as high (9). Toward the end of the news coverage of the crisis in Flint, it was announced that there was a water crisis in schools in Newark, New Jersey, where levels of lead coming from school water sources often exceeded the levels of lead found in Flint (10). Then, in the spring of 2017, parents in an Ocean County, New Jersey, grade school were sent an email message discussing some failure of school water sources because of lead. Buried within the pages of scientific documentation was a grade school water source that was reported to have 30 times more lead than the Flint water (850 ppb) (see Figure 1).
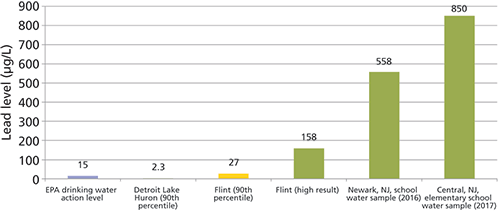
Figure 1: Comparison of lead levels found in Flint water samples and New Jersey school water sources in 2015–2017 (µg/L).
CLICK FIGURE TO ENLARGE
Public Awareness
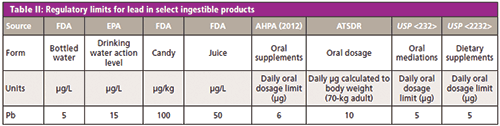
Most scientists and experts agree that the most positive thing to come from the crisis in Flint is a renewed public awareness focused on lead exposure and the contamination from lead products and legacy infrastructures. Over the past decade, regulatory agencies have started to either issue or reexamine lead limits for common exposure threats (air, water, soil, and dust) and daily ingestible products (food, medications, supplements, water, and food) (see Table II).
CLICK TABLE TO ENLARGE
Food has not been considered to be the main source of lead intake since the mid-1990s because of the banning of lead solder in canned food and lead seals. But, that does not negate the fact that despite the precautions to limit lead, high amounts are still found in common foods and nutraceuticals. Some of this lead contamination is natural, through bioaccumulation of lead from contaminated soil and water. Lead can accumulate in dense tissues like bones, organs, roots, or seeds and become condensed into dehydrated or processed products (such as nuts, spices, dried fruit, and bone meal).
Foodstuffs
A popular food reported to contain lead is chocolate. Cacao is often grown in regions with historical heavy metal pesticide application. Metals can accumulate in the seed pod used to created chocolate, thereby potentially increasing heavy metal contamination. In 2005, Rankin and colleagues reported lead levels ranging from 0.5 µg/kg to 230 µg/kg in chocolate and cacao samples (11). Spex CertiPrep examined several different types and forms of chocolate and found levels from 10 µg/kg in chocolate liquor to 75 µg/kg in a dark chocolate using inductively coupled plasma–mass spectrometry (ICP-MS) (13). The overall lead concentrations in the chocolate products were all higher than the concentration of lead found in the majority of Flint water samples (Figure 2). The difference is seen in the dosage where the Spex CertiPrep chocolate sample would provide 3 µg of lead in a typical 40 g chocolate bar whereas an 8-oz glass of Flint water would provide about 6 µg of lead. Two chocolate bars would equate to the same amount of lead that was found in a glass of Flint water (Figure 3).
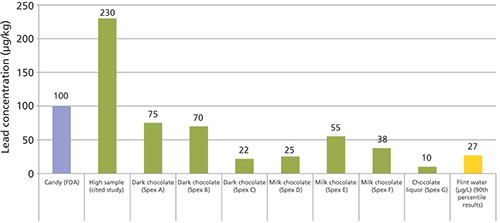
Figure 2: Lead concentration (µg/kg) found in chocolate samples and FDA limit for candy.
CLICK FIGURE TO ENLARGE
Fresh Seafood
A second popular food product exposed to metals contamination is fish. A study of different types of commercially purchased fish showed that although mercury usually is the metal of primary concern in seafood, lead can also be a significant contaminant. Our fish samples, which included tuna, salmon, and swordfish, contained between 7 and 170 µg/kg of lead. The highest lead was found in the dark tuna (170 µg/kg), which was six times higher than the concentration in the cited 27-µg/kg, 90th percentile Flint sample. This level of lead in seafood would expose a normal adult to almost 40 µg of lead in an 8-oz uncooked portion. This amount would be the equivalent of drinking 1.5 L of the 27-ppb Flint water sample (see Figure 3).
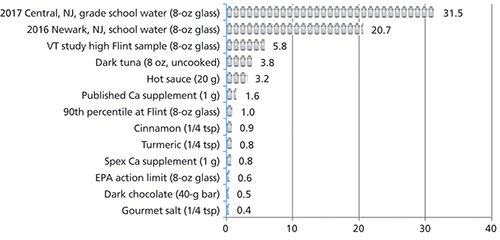
Figure 3: The equivalent volume of Flint water (8-oz glasses) needed to equal the Pb (µg) in a daily dose of ingestible products.
Calcium Supplements
Lead can have an affinity for calcium binding sites, meaning that calcium tissues and products made from them (dairy, bone meal, and calcium supplements) can accumulate high levels of lead. A survey of studies on calcium supplements in 2000 by Scelfo and Flegal in Environmental Health Perspectives described a range of lead levels in calcium supplements up to almost 10,000 µg/kg of supplement (12). At Spex CertiPrep, we conducted a small study on elements in commercial calcium supplements and found lead levels ranging from 170 µg/kg to a high of 4800 µg/kg (13). Therefore a 1 g dose of a calcium supplement would add about 5 µg to an adult's daily exposure of lead, which would be the equivalent to drinking about 6 oz of the Flint water (Figure 4).
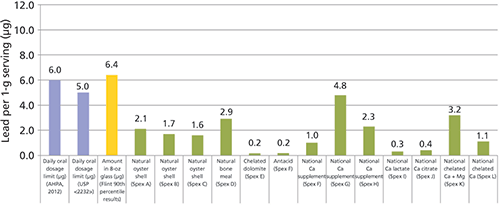
Figure 4: Lead concentration in calcium supplements (µg) in a daily 1-g serving.
CLICK FIGURE TO ENLARGE
Adulteration of Food Materials
An important route of exposure to lead from food comes from intentional adulteration or accidental contamination of food products. Incidents have occurred over the last few years where imported candy from Mexico was found to contain high lead levels. The lead was attributed to several sources ranging from cooking pots and printing ink to the inclusion of spices that were either contaminated or adulterated with lead. The adulteration of food is a growing problem. Billions of dollars are spent each year on adulterated food products such as chocolate, honey, spices, and supplements. Usually, the adulteration does not cause any significant illness but there have been cases where the products have caused illnesses and deaths. In 1994, dozens of people fell ill and several died from Hungarian paprika adulterated with lead oxide.
Common Spices
Spices are one of the most expensive world commodities, which creates motivation for adulteration. Spices can be adulterated or counterfeited in a variety of means from bulking agents and dyes to substitution by look-alike counterfeits. Spex CertiPrep conducted a study on the contamination and adulteration of common spices where we found that many spices including black pepper, cinnamon, turmeric, and chili peppers contained very high levels of lead and other heavy metals (13). The average amount of lead across all the tested spices was about 1600 µg/kg. The highest levels of lead were found in the cinnamon (2800 µg/kg), turmeric (2700 µg/kg), and red pepper samples (2200 µg/kg) (see Figure 5).
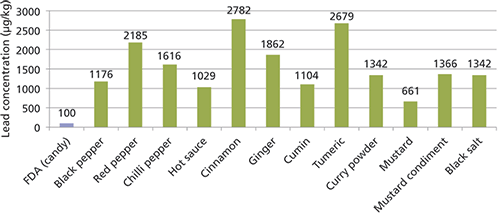
Figure 5: The concentration of lead found in common spices (µg/kg).
Despite the high concentrations of lead in the spices, in general, the average person consumes a small amount of any one spice; therefore, daily exposure is fairly low. A quarter teaspoon of cinnamon (1.5–2 g) would result in 4–5 µg of lead in a day equivalent to drinking almost a glass of the average Flint water. However, more alarming was the daily exposure to lead from some spice products such as hot sauce. Hot sauce packets were obtained from a Chinese food restaurant. The concentration of lead found in these packets was 1000 µg/kg (see Figure 5). But, the typical amount of hot sauce used was between one and two packets. Each packet contained 10 g of hot sauce, making the actual lead dosage from two packets to be 20 µg, which was the equivalent of drinking 0.75 L of the average Flint water.
The problem of our exposure to lead is a lack of a consensus for tolerable overall intake levels for lead from all sources on a daily, weekly, or monthly basis. Many regulating bodies, while issuing individual limits for circumstances, incidents of exposure, or products, stop short of proposing overall total exposure limits. In the absence of definitive guidelines, it becomes more important to understand lead exposure from the situations or products that are chosen in daily life. If knowing that Chinese hot sauce is going to add 20 µg of lead to your diet (refer to Table III), or that a piece of fish for dinner has almost the same amount of lead as four glasses of the Flint water (see Figure 3), perhaps a different choice might be made.
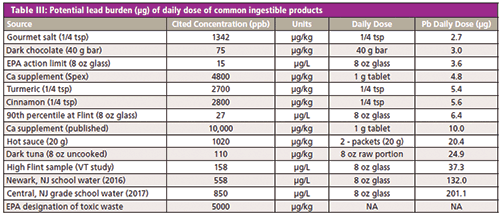
CLICK TABLE TO ENLARGE
Final Thoughts
The story of lead in our daily lives has not been properly addressed with the latest public health crisis in Flint. The real story of lead is our daily exposure and burden from all the sources of lead we come into contact with each day. It is the story of all the states with high lead levels in drinking water. It is the story of all the schools in the United States with outdated lead plumbing, exposing school children to lead in the classroom. Although the high amounts of lead publicized recently puts a face on the problem, it does not adequately show that while a tragic public health event occurred, it did not occur in a vacuum. The crisis also did not show that there are many sources of lead we are exposed to each day. The average person is exposed to more lead than what is in our water. Lead is found in the air and dust we inhale and the food we ingest, in addition to the water we drink. The amount of lead exposure from our drinking water should also be combined with all the other sources to have a better understanding of the total exposure of lead in our daily lives.
References
(1) I. Tapsoba, S. Arbault, P. Walter, and C. Amatore, Anal. Chem. 82(2), 457–460 (2010). https://doi.org/10.1021/ac902348g.
(2) National Research Council (U.S. Ed.). Lead in the Human Environment: A Report (Washington, D.C., National Academy of Sciences, 1980).
(3) mcs-2017-lead.pdf. (n.d.). Retrieved from https://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2017-lead.pdf.
(4) H.W. Mielke, Lead Perspective 30(3), 231–242 (2008).
(5) K. Schwartz, "The Spatial Distribution of Lead in Urban Residential Soil and Correlations with Urban Land Cover of Baltimore, Maryland," thesis, Rutgers University, New Brunswick, N.J. (2010).
(6) C. Ingraham, "This is How Toxic Flint's Water Really Is," Washington Post (January 15, 2016). Retrieved from https://www.washingtonpost.com/news/wonk/wp/2016/01/15/this-is-how-toxic-flints-water-really-is/.
(7) D.C. Bellinger, N. Engl. J. Med. , 374(12), 1101–1103 (2016). https://doi.org/10.1056/NEJMp1601013.
(8) D. Gusovsky, "America's Water Crisis Goes Beyond Flint, Michigan," CNBC (March 24, 2016). Retrieved August 29, 2017, from https://www.cnbc.com/2016/03/24/americas-water-crisis-goes-beyond-flint-michigan.html.
(9) M.B. Pell and J. Schneyer, "The Thousands of U.S. Locales Where Lead Poisoning Is Worse Than in Flint," Off the Charts (December 19, 2016). Retrieved August 29, 2017, from http://web.archive.org/web/20170502091728/https://www.reuters.com/investigates/special-report/usa-lead-testing/.
(10) S. Ganim and L. Tran, "How Flint, Michigan's Tap Water Became Toxic," CNN (January 13, 2016). Retrieved August 29, 2017, from http://www.cnn.com/2016/01/11/health/toxic-tap-water-flint-michigan/index.html.
(11) C.W. Rankin, J.O. Nriagu, J.K. Aggarwal, T.A. Arowolo, K. Adebayo, and A.R. Flegal, Environ. Health Perspect. 113(10), 1344–1348 (2005). https://doi.org/10.1289/ehp.8009.
(12) A.R. Flegal and G.M. Scelfo, Environ. Health Perspect. 108, 309–319 (2000).
(13) https://www.spexcertiprep.com/knowledge-base/appnotes-whitepapers.
Patricia Atkins

Patricia Atkins is a graduate of Douglass College and Rutgers University in New Jersey. She started in the chemical industry with a position as a QC Chemist and lab supervisor for Ciba Specialty chemicals. Patricia later accepted a position conducting research and managing an air pollution research group within Rutgers University's Civil & Environmental Engineering Department. In 2008, Patricia joined SPEX CertiPrep as an application scientist for the certified reference material's division. She spends her time researching industry trends and developing new reference materials.

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Applications of Micro X-Ray Fluorescence Spectroscopy in Food and Agricultural Products
January 25th 2025In recent years, advances in X-ray optics and detectors have enabled the commercialization of laboratory μXRF spectrometers with spot sizes of ~3 to 30 μm that are suitable for routine imaging of element localization, which was previously only available with scanning electron microscopy (SEM-EDS). This new technique opens a variety of new μXRF applications in the food and agricultural sciences, which have the potential to provide researchers with valuable data that can enhance food safety, improve product consistency, and refine our understanding of the mechanisms of elemental uptake and homeostasis in agricultural crops. This month’s column takes a more detailed look at some of those application areas.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.