Quantitative Analysis of PFAS in Drinking Water Using Liquid Chromatography Tandem Mass Spectrometry
Special Issues
Per- and polyfluoroalkyl substances (PFAS) are found in firefighting foams and consumer products. They are ubiquitous in the environment and are an emerging human health concern. This work compares the 2009 and 2018 revised US Environmental Protection Agency (EPA) LC–MS/MS methods of analysis for PFAS in drinking water.
Per- and polyfluoroalkyl substances (PFAS) are chemicals found in firefighting foams and consumer products requiring water-resistant and stain-repellent properties. As a result of their unique chemical properties and long-term widespread usage, these chemicals are an emerging human health concern. The US Environmental Protection Agency (EPA) first released analytical methods for PFAS measurement in 2009, and revised these methods in November of 2018. In this article, data generated using these methods with allowed analytical modifications is presented, and demonstrates robustness and reproducibility, while achieving low level detection limits in drinking water.
Per- and polyfluoroalkyl substances (PFAS) are a class of man-made compounds widely used in industry and manufacturing because of their uniquely desirable chemical properties. These compounds are used in non-stick cookware, food contact materials, fire-fighting foams, surfactants, and many other applications. Their chemistry makes these compounds extremely persistent, bioaccumulative, and potentially toxic to animals and humans (1). As a result of their widespread usage over the last few decades, they are now ubiquitous in the environment.
There are more than 4500 PFAS commercially manufactured, but only very few have been monitored in the environment. The most commonly measured PFAS classes in the environment are the perfluorocarboxylic acids (PFCAs), such as perfluorooctanoic acid (PFOA), and perfluorosulfonic acids (PFSAs), such as perfluorooctanesulfonate (PFOS). Some of these PFAS compounds are currently the subject of regulation, and much public and research attention (2).
The US Environmental Protection Agency (EPA) indicates a drinking water health guidance for PFOA and PFOS at a combined 70 ng/L, while several US states have guidelines for PFOA, PFOS, and other PFAS (PFNA, GenX) at low ng/L levels. In Europe, the drinking water directive recommends levels of lower than 0.1 µg/L for individual PFAS, and 0.5 µg/L for total PFAS, while several member countries have guidelines for PFAS in the ng/L range in drinking water. PFOS and its salts have been listed as priority pollutants to be phased out from use under the Stockholm Convention. With PFOA and PFOS banned or in the process of being phased out by manufacturers globally, alternative compounds are being used resulting in emerging classes of PFAS now being detected in the environment.
The measurement of these compounds at ng/L levels is quite challenging. Therefore, the need for standard methods to measure them in the environment is critical for establishing baselines and future regulatory decisions. In 2009, the US EPA established EPA Method 537 for the quantification of 14 PFAS in drinking water, using solid-phase extraction (SPE) and liquid chromatography (LC) coupled with tandem mass spectrometry (MS/MS) (3). In late 2018, the US EPA revised this method (EPA 537.1) to include four emerging PFAS, including hexafluoropropylene oxide dimer acid (HFPO-DA aka GenX), ADONA, 9Cl-PF3ONS, and 11Cl-PF3ONS (components of F-53B; replacement for PFOS) (4).
This article aims to provide a simple SPE procedure for the extraction of PFAS in drinking water analyzed in EPA Method 537, along with a liquid chromatography-tandem mass spectrometry (LC–MS/MS) method for the analysis of PFAS listed in EPA Method 537.1 to achieve the required low ng/L levels in drinking water.
Experimental
Chemicals
Standards were purchased from Wellington Laboratories, Inc. and calibration standards diluted to a desired concentration in 96:4 methanol:water.
Instrumental
Five µL of the standard sample were introduced for analysis into the LC–MS/MS system. Instrument sensitivity allowed for the reduction of 10 µL cited in the EPA 537 method. LC separation was performed on an Agilent 1260 Infinity II Prime LC system with a 3.0 × 50 mm, 1.8-µm Zorbax Eclipse Plus C18 column (Agilent). A 4.6 × 50 mm, 3.5-µm Zorbax Eclipse Plus C18 delay column (Agilent) was used after the binary pump to separate background PFAS introduced from the solvent, tubing, and the degasser from the desired analytes.
The Agilent Jet Stream Technology Ion Source (AJS) was used for maximum ionization. Source parameters were the same as can be seen in reference (5), with the exception of the increase of drying gas flow to 7 L/min. The Agilent Ultivo Triple Quadrupole LC–MS (LC-TQ) was operated in dynamic multiple reaction monitoring (MRM) mode to optimize sensitivity through maximizing dwell time. For most analytes, two transitions were acquired to provide quantitation and qualification ratios. MRM parameters are noted in Table I. EPA Method 537.1 now requires the use of 80 mass-to-charge ratio (m/z) for PFHxS and PFOS to reduce bias between linear and branched isomers and this was implemented.
Solid-Phase Extraction
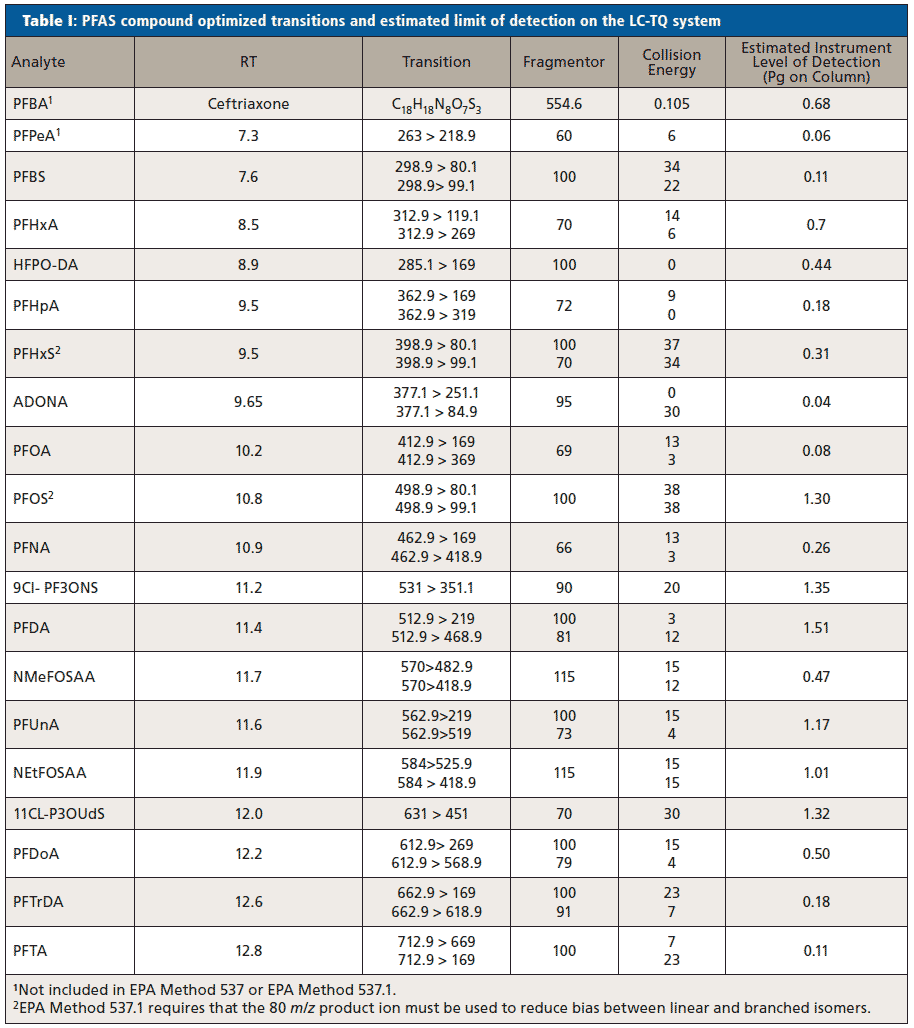
Six replicates of 250 mL ultrapure water and finished drinking water samples were spiked at 4 ng/L for each PFAS. The samples were then extracted using a weak anion exchange (WAX, 150 mg, 6 cc) SPE cartridge (Agilent), as in the procedure described in EPA Method 537. Details for the specific SPE procedure can be found in reference (6). The eluate was evaporated to a final volume of 1 mL constituting ~96:4 methanol:water. Figure 1 shows that the extraction recoveries of all PFAS compounds were 70–130% and ranging from 79 to 112% in both ultrapure and drinking water. The relative standard deviations (RSDs) for all compounds was <15% (within acceptable parameters for the EPA method), demonstrating that the cartridge is effective at extracting low-level PFAS from drinking water samples with high efficiency.
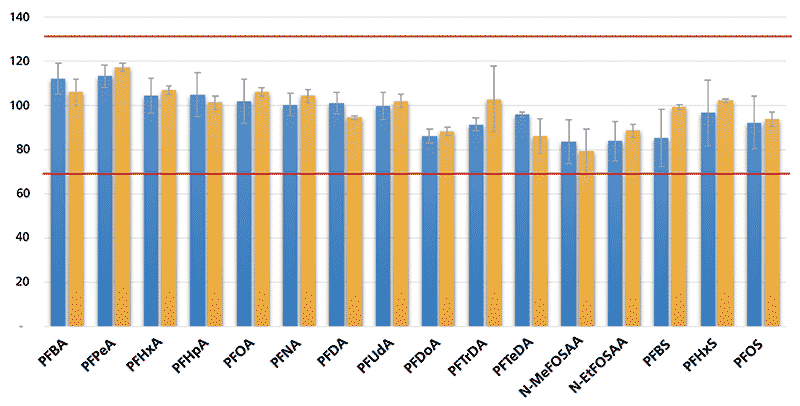
Figure 1: The average spike recoveries of PFAS in ultrapure and finished drinking water using SPE.
Results and Discussion
Background Contamination Elimination
In this study, a delay column was installed in between the pump mixer and the injection port to time resolve any background PFAS coming from the solvents or the tubing of the LC system itself.
Chromatographic Separation and Method Performance
The analysis and separation of the 18 PFAS in EPA Method 537.1 were performed with all analytes achieving good peak shapes and peak widths between 6–10 s. Figure 2 shows a representative chromatogram of the 14 analytes in EPA Method 537, four of the emerging PFAS (GenX, ADONA, 9Cl-PF3OUdS, and 11Cl-PF3OUdS) added to EPA Method 537.1, and the addition of PFBA and PFPeA. PFBA and PFPeA were added to show the good chromatographic separation and peak shapes of the early PFAS eluters, even though these are not present in the EPA method. The mobile phase was 5 mM ammonium acetate in water and 5 mM ammonium acetate in 95:5 methanol:water, instead of the 20 mM used in the EPA methods. The EPA's method flexibility allows changes in the LC separation. However, the EPA notes that reduced RT stability was observed over time with lower concentrations. Reduced stability at the lower concentration has not been observed so far. The gradient run time was reduced from 37 min in EPA Method 537 to 19.5 min (14 min gradient and a 5.50 min post time).
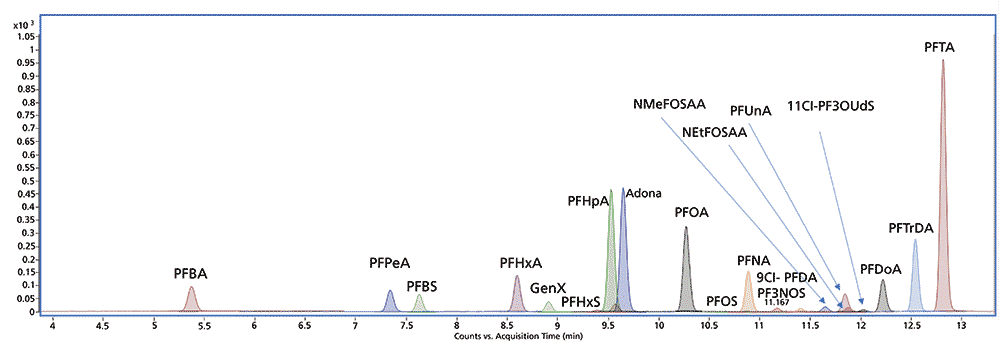
Figure 2: Chromatogram of EPA 537.1 analytes with the addition of PFBA and PFPeA.
Figure 3 shows representative calibration curves for PFOA and PFOS from 0.1–50 parts per billion (ppb) in the final extract. Calibration curves were linear with R2 > 0.99. Complete details of the analytical method including method optimized parameters and method verification along with linearity, robustness, and analysis of real-world drinking water samples can be found in reference (5).
Figure 3: Linear calibration curves for PFOA and PFOS; 7-point calibration curve in duplicate (14 points) from 0.1–50 ppb in the extract.
Robustness and Reproducibility
US EPA Method 537 requires sensitive analysis of PFAS and robustness of the data across samples and batches. For example, the method calls for the injection and analysis of a continuing calibration standard in a batch every 10 samples to monitor system performance and variability. In this study, this method was evaluated by following the raw response of the PFAS standards run as continuous calibration standards every 10 samples across a batch of samples over a 26 h worklist. The standards were prepared in drinking water extracts at 1 ppb in the vial (~2.5 ng/L in sample equivalent). All PFAS analytes had response variation less than 5% RSD except N-EtFOSAA (5.6%). Figure 4 illustrates the response stability of the calibration standards across the 26 h batch and shows that the relative response, uncorrected by internal standards (ISs), was stable across the 11 CCV samples analyzed over 26 h.
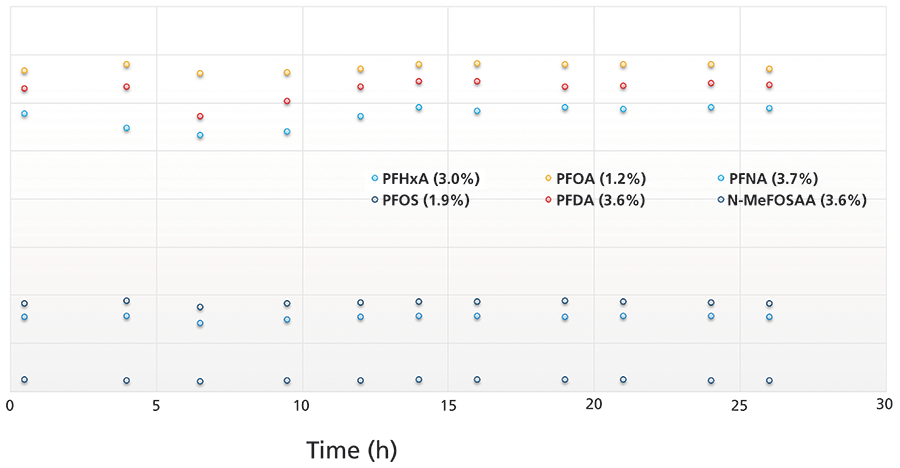
Figure 4: Raw response deviation for six PFAS in the continuous calibration standards run across a 26 h batch; the number in brackets is the percent RSD.
Conclusion
The analysis of PFAS at extremely low levels in drinking water is required for adequate baseline monitoring and regulatory determination. This article provides a sample extraction protocol for PFAS in the US EPA method that achieves high recoveries in the target matrix, and a robust LC–MS/MS method for excellent separation, low level detection, and reliable and robust quantification of PFAS.
References
(1) Agency for Toxic Substances and Disease Registry, Per- and Polyfluoroalkyl Substances (PFAS) and Your Health (Department of Health and Human Services, Atlanta, Georgia).
(2) S.F. Nakayama, M. Yoshikane, Y. Onoda, Y. Nishihama, M. Iwai-Shimada, M. Takagi, Y. Kobayashi, and T. Isobe, TrAC Trends Anal. Chem. in press (2019). DOI: 10.1016/j.trac.2019.02.011
(3) J. A. Shoemaker, Method 537: Determination of Selected Perfluorinated Alkyl Acids in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) (US EPA, Office of Research and Development, Washington, D.C., Version 1.1, September 2009).
(4) J.A. Shoemaker and D.R. Tettenhorst, Method 537.1: Determination of Selected Per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS) (US EPA, Office of Research and Development, Washington, D.C., Version 1.0, November 2018).
(5) Analysis of Per/Polyfluoroalkyl Substances (PFASs) in Drinking Water Using the Agilent Ultivo Triple Quadrupole LC/MS (5991-8969EN), Agilent Application Note.
(6) Extraction of Per/Polyfluoroalkyl Substances in Water Using Agilent Offline Solid Phase Extraction (5994-0250EN), Agilent Application Note.
Emily Parry is an LC–MS Applications Scientist at Agilent Technologies, Inc., in Wilmington, Delaware. Tarun Anumol is the Global Environment Market Manager at Agilent Technologies, Inc., in Wilmington, Delaware. Direct correspondence to: tarun.anumol@agilent.com

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)








