Raman Spectroscopy of Supported Lipid Bilayer Nanoparticles
Special Issues
Raman spectroscopy (weak H2O Raman scattering) has been a tool of choice for investigating aqueous lipid suspensions. Recently, there has been interest in supported lipid bilayers (SLBs), where lipids are believed to have fluidities similar to those of free vesicles, and thus have been investigated for applications such as sensors and drug delivery vehicles. Here, Raman spectra of two lipid SLBs on SiO2 nanoparticles were obtained. With decreasing nanoparticle size, or for the same nanoparticle size and longer alkyl chain length, the lipids became increasingly interdigitated compared with the normal bilayer structure.
Raman spectroscopy (weak H2O Raman scattering) has been a tool of choice for investigating aqueous lipid suspensions. Recently, there has been interest in supported lipid bilayers (SLBs), where lipids are believed to have fluidities similar to those of free vesicles, and thus have been investigated for applications such as sensors and drug delivery vehicles. Here, Raman spectra of two lipid SLBs on SiO2 nanoparticles were obtained. With decreasing nanoparticle size, or for the same nanoparticle size and longer alkyl chain length, the lipids became increasingly interdigitated compared with the normal bilayer structure.
Nanoparticles are now used in wide range of diverse applications that include drug delivery vehicles, sensors, colloidal dispersions and nanocomposites. For all of these applications, the interphase region, where the surface of the nanoparticle interacts with the surrounding material, becomes critically important and can depend on factors such as the charge, polarity, and degree of hydrophobicity of the nanoparticle. The nanoparticle itself can be composed of organic polymers or inorganic materials such as silica (SiO2). The high surface-to-volume ratio of nanoparticles can be exploited to improve bonding between nanoparticles and the surrounding resin or to increase the dynamic range in sensor applications. Alternatively, the high surface-to-volume ratio can be exploited to investigate the interphase region and the chemical or physical interactions between inorganic surfaces and organic materials. Fourier transform–infrared (FT-IR) and Raman spectroscopy are often employed to obtain information on chemical composition and interactions between materials, conformational properties of molecules, and their state of order. Attenuated total reflectance (ATR)–FT-IR, surface-enhanced Raman spectroscopy, and nonlinear optical techniques such as second harmonic generation can be used to obtain information on surface properties, molecules at surfaces, and their interactions. Raman spectroscopy has recently been used to measure lipid multibilayers on planar supports (1).
Here, we use conventional Raman spectroscopy to investigate the conformational order, packing, and phase transition behavior of supported lipid bilayers (SLBs) of zwitterionic lipids on silica (SiO2) nanoparticles. Raman spectroscopy is particularly suited to this study because it is well known that both water and SiO2 are weak Raman scatterers but strong IR absorbers. The high surface-to-volume ratio is exploited to investigate single and multiple bilayers of lipids on SiO2 nanoparticles ranging in size from 4–5 to 100 nm in diameter. Since these are monolithic, rigid particles, the effects of curvature on the lipid gel-to-liquid crystal phase transition temperature, and the conformation and packing of the chains on the nanoparticle as a function of size, can be investigated. These investigations are aided by the extensive body of previous literature that relates spectral features in the skeletal and CH bending–stretching regions of alkyl chains to the ratio of trans/gauche conformers and to lateral packing of the chains in both the gel and liquid crystalline phases of lipids (2,3).
In this work, we present an effect that has not been previously observed spectroscopically, using the conformationally dependent bands in the optical skeletal region and bands in the methylene stretching region that are sensitive to lateral packing order. In particular, zwitterionic 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) SLBs were shown to form increasingly interdigitated structures as the nanoparticle size decreased. A vibration at 1122 cm-1 attributed to alkyl chains with a single gauche rotation of the terminal methyl group near the bilayer center — a conformation that permitted better hydrophobic packing of the chains on curved surfaces — was recorded in the Raman spectra of these SLBs.
Experimental
The lipids used in this study, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), were obtained from Avanti Polar Lipids (Alabaster, Alabama). Snowtex colloidal silica (SiO2) beads with nominal diameters of 4–6, 10–20, 20–30, and 40–50 nm were kindly donated by Nissan Chemical Industries, Ltd. (Tokyo, Japan). Multilamellar vesicles were prepared by first dissolving appropriate amounts of the lipids or lipid mixtures in chloroform, followed by drying with N2/vacuum to form films. The films were redispersed in a 0.1 M, pH 8.0 buffer made from Na2HPO4•7H2O, NaH2PO4•H2O (PBS), and 75 mM NaCl, and incubated for 2 h above the gel–liquid crystal transition temperature, Tm, of the lipids. Small unilamellar vesicles (SUVs) or large unilamellar vesicles (LUVs) were obtained from multilamellar vesicles by subjecting them to five freeze–thaw cycles followed by extrusion (Avanti Mini-Extruder from Avanti Polar Lipids) using a polycarbonate filter with pore sizes of 50 or 100 nm. SUV sizes of approximately 60 and 100 nm were measured by dynamic light scattering (DLS) (4). Adsorption of vesicles onto the beads was accomplished by mixing the SUV and SiO2 dispersions and incubating at temperatures above the Tm of the respective lipids for 2 h (shown schematically in Figure 1). The amount of lipid, in terms of its surface area (SASUV) required to form a single bilayer around the beads (SAsilica) is SASUV/SAsilica = 1. Here, the suspensions were mixed with excess lipid, SASUV/SAsilica > 2, and centrifuged at 3900 rpm (Marathon 3200 centrifuge, Fisher Scientific, Pittsburgh, Pennsylvania) and the supernate decanted. Additional water was added to the pellet, and the centrifuge–washing steps repeated three times. This procedure ensured that the SiO2 nanoparticles were fully covered with lipid, but that only a single bilayer remained, as observed by transmission electron microscopy (TEM) and cryo-TEM data (Figure 1). After the last centrifugation, the pellet was redispersed in 300 μL D2O for the Raman experiments or HPLC-grade water for nano-differential scanning calorimetry (nano-DSC) experiments.
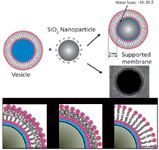
Figure 1: Schematics of formation of a supported lipid bilayer on SiO2 nanoparticles, and interdigitated, partially interdigitated, and bilayer structures. Cryo-TEM image of a supported lipid bilayer on a 100-nm nanoparticle.
Instrumentation
Raman spectroscopy analysis was performed using a micro-Raman spectrometer (RM 1000, Renishaw, Gloucestershire, United Kingdom) with a 514.5-nm air-cooled Ar ion laser source and an 1800-lines/mm grating polychromator with RenCam CCD detection, providing a resolution of 1 cm-1. The laser source was focused on the sample nanoparticle suspension through a long working distance, 50× objective, to a spot diameter of approximately 2 μm, and the Raman signal from the SLBs was collected in backscattering geometry. The samples were studied in standard aluminum pans for DSC (TA Instruments, New Castle, Delaware). A THMS600 heating stage (Linkam Scientific Instruments, Surrey, United Kingdom) was used for temperature control with 0.1 °C accuracy of the nanoparticle suspensions during phase transition studies. In those cases, Raman spectra were recorded after allowing thermal equilibrium to be achieved at every temperature point for 10 min.
The acquisition time for Raman spectra was 10–20 min, depending on the strength of the Raman signals, until a satisfactory signal-to-noise ratio was achieved. It was found that SLBs in smaller particle size suspensions, because of their higher surface areas per unit volume, produce much stronger Raman signals. Therefore, the longer accumulation times were needed in the case of suspensions of bigger diameter nanoparticles and also when collecting spectra in the fingerprinting region 650–1800 cm-1 where the band intensities of SLBs were much weaker than in the stretching region (2700–3200 cm-1). Possible laser-heating effects during the Raman spectral collection were eliminated by limiting the argon laser power density to levels where it was proven that the SLB spectra are not affected by prolonged irradiation. Data analysis was performed using the Renishaw Wire 2.0 software.
Nano-DSC measurements were obtained on a TA Instruments Nano DSC-6300. Samples were scanned at heating–cooling rates of 1 °C/min, using 1–2 mg total lipid in the analyzed samples.
Results and Discussion
A representative spectrum with band assignments for DPPC SLBs is shown in Figure 2. The most intense bands in the spectra of molecules including alkanes and lipids with alkyl groups are the CH stretching modes, and therefore there has been much research focused on their interpretation. This spectral region is shown for DSPC multilamellar vesicles and for DSPC SLBs on the 100, 40–50, 20–30, 10–20, and 4–5 nm SiO2 in Figure 3. While the signal-to-noise ratio decreases for DSPC on the largest size SiO2 nanoparticles, the same spectral features are observed for both multilamellar vesicles and SLBs. The CH3 symmetric stretching modes appear at 2871 cm-1, with a Fermi resonance (FR) component at 2938 cm-1 (r+FR ). The peaks at 2952 and 2964 cm-1 are the CH3 out-of-plane and in-plane methyl antisymmetric stretches (r-) (5). The methylene vibrations at approximately 2852, 2884, 2900, and 2928 cm-1 are sensitive to conformational changes as well as intermolecular interactions of the alky chains of lipids. The νa(CH2) antisymmetric stretch (d-) is coupled to rigid rotations–torsional vibrations (6,7), so that it broadens considerably with temperature, and increases continuously in frequency, from 2884 cm-1 to 2898 cm-1 as gauche conformers are introduced (6). The νs(CH2) symmetric stretch (d+) contains three components, centered at 2852 cm-1 (d+FR), 2900 cm-1 (d+FR), and 2928 cm-1 (d+FR), because of extensive Fermi resonance interactions with overtones of the bending modes, and is affected by intra- and inter-molecular interactions.

Figure 2: Raman spectrum of supported lipid bilayers of DPPC, showing structure of DPPC and band assignments. The intensity in the 650â1800 cm-1 region is shown magnified approximately 5Ã of the original for clarity.
The temperature dependence of the CH2 vibrations for DPPC on the 40–50 nm SiO2 nanoparticles is shown in Figure 4. As is observed for multilamellar vesicles, with increased temperature, the antisymmetric CH2 stretch frequency, νa(CH2) at 2884 cm-1 increases to 2890 cm-1 and its intensity is reduced compared with one component of the symmetric CH2 stretch, νsFR(CH2) at 2845 cm-1. The νsFR(CH2) vibration increases from 2847 to 2852 cm-1 with increasing temperature. The νsFR(CH2,CH3) symmetric stretch at 2922–2935 cm-1 (also contains CH3 component) increases in intensity with respect to both the νsFR(CH2) = 2845 cm-1 and νa(CH2) = 2884 cm-1 bands.
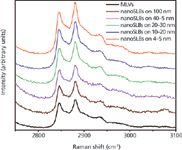
Figure 3: C-H region for DSPC multilamellar vesicles (MLVs) and DSPC supported lipid bilayers (SLBs) on SiO2 nanoparticles of different diameters.
Spectroscopic determination of phase transition temperatures (Tm) can be derived from intensity ratios of bands in the CH stretching region (8,9). An intensity ratio often used is I2845/I2884, or alternatively, a lateral order parameter (Slateral) derived from this ratio, Slateral = [(I2845/I2884) – 0.7]/1.5 (3). Here, S = 1 indicates the highest possible order and S = 0 indicates no order (not necessarily the lowest possible), and is based on a comparison of intensity differences in crystalline and liquid hexadecane (3). Both parameters semiquantitatively reflect the lateral packing of the chains. Plots of Slateral for DSPC on 40–50 and 4–5 nm SiO2 nanoparticles are presented in Figure 5, along with nano-DSC thermograms of the phase transition temperatures measured calorimetrically. The measurements of Tm based on Raman spectroscopy and nano-DSC are in reasonable agreement, and both transitions also are broader than previously observed for multilamellar vesicles.

Figure 4: C-H stretching modes of supported lipid bilayers of DPPC on 40â50 nm SiO2 nanoparticles as a function of temperature.
The lateral packing of the alkyl chains is compared as a function of nanoparticle size in Figure 6 (left) for both DPPC and DSPC. In both cases, lateral packing is observed to increase with decreasing nanoparticle size. This initially seems counterintuitive. However, if the polar headgroups of the inner leaflet of the bilayer pack as on a planar surface, then there is increasing free volume as the curvature of the solid support increases (that is, nanoparticle size decreases). One way to decrease this free volume and increase hydrophobic interactions is for lipid interdigitation, or partial interdigitation, to occur, as shown schematically in Figure 1. Previous investigations have indicated that curvature can induce interdigitation in DPPC vesicles (10) and SLBs (4), and Raman data have shown that there is increased lateral order for interdigitation compared with normal bilayer systems (11). For each nanoparticle size, the lateral order, and thus the interdigitation, is greater for the longer alkyl chain DSPC (18 carbons) compared with DPPC (16 carbons). For a given nanoparticle size, the increased free volume would be greater for the longer length alkyl chain, supporting the view that increased interdigitation would occur.

Figure 5: (Top) Lateral order parameter as a function of temperature for SLBs on 40â50 and 4â5 nm SiO2. (Bottom) Corresponding nano-DSC thermograms showing calorimetric phase transition temperatures.
Of further interest is the C-C skeletal region of the alkyl chains, shown in Figure 7 for DPPC multilamellar vesicles and SLBs, where in saturated lipids, three bands are observed at 1060, 1000, and 1127 cm-1 in the low temperature gel phase. The 1060/1140 pair is attributed to all trans segments, and the band at 1000 cm-1 to chains with a single gauche defect, although the placement of the defect along the chain is not specified (12). In the high temperature liquid crystalline phase, the intensity of the 1064- and 1130-cm-1 bands decreases, the 1064-cm-1 band shifts up in frequency, the 1130-cm-1 band shifts down in frequency, and the 1101-cm-1 band shifts and merges with a new broad intense band at 1090 cm-1 (not shown). The amount of alkyl chain disorder is often taken as the relative number of gauche/trans conformers, measured by their Raman intensity ratios I[ν(C-C)G]/I[ν(C-C)T] = I1090/I1060. This ratio is plotted versus lateral order of the DPPC and DSPC alkyl chains for SLBs as a function of nanoparticle size in Figure 6 (right). For both the DPPC and DSPC, lateral packing increases as the relative number of trans conformers increases, as expected, except for the smallest nanoparticle size. In addition, lateral order and the relative number of trans bonds is greater for DSPC compared with DPPC at every nanoparticle size.
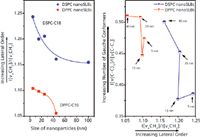
Figure 6: (left) Lateral order of DPPC and DSPC alkyl chains measured by Raman scattering intensity ratio, I[Ï a(CH2)]/I[Ï s(CH2)] = I2884/I2845, as a function of nanoparticle size. (Right) Ratio of gauche/trans conformers in DPPC and DSPC alkyl chains measured by Raman scattering intensity ratio, I[Ï (C-C)G]/I[Ï (C-C)T] = I1090/I1060, as a function lateral order of the alkyl chains for SLBs of different nanoparticle sizes.
What is of further interest for the DPPC and DSPC SLBs is the presence of a band (or shoulder band) at 1122 cm-1, attributed to alkyl chains with a single gauche rotation of the terminal methyl group near the bilayer center, as shown for DPPC in Figure 7. The band is not observed at RT for DPPC multilamellar vesicles, but is observed as a shoulder for the LUVs. The difference between LUVs and SUVs is just their size, both of which are significantly smaller than multilamellar vesicles. This suggests that the 1122-cm-1 band has to do with the packing of the alkyl chains. One possibility is that as free volume increases with decreasing size of the vesicles (given that the multilamellar vesicles have almost planar structures with respect to the size of the individual lipids), the ends of the chains kink to fill the free volume that exists between the chains towards the center of the bilayer. The appearance of the 1122-cm-1 band is apparent in all of the SLBs, suggesting that on these geometrically fixed structures, kinking of the chains towards the center of the bilayer is another mechanism, along with interdigitation, that fills the free volume between the chains.
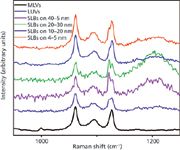
Figure 7: C-C skeletal vibrations for DPPC multilamellar vesicles (MLVs), large unilamellar vesicles (LUVs) and DPPC supported lipid bilayers (SLBs) as a function of SiO2 nanoparticle diameter. There are characteristic vibrations at 1060, 1090, 1121, and 1127 cm-1.
Conclusions
Raman spectroscopy was shown to be a powerful technique in which to study the interface region of inorganic nanoparticles, particularly ones such as SiO2 that are not strong Raman scatterers. Because water is also a weak Raman scatterer, it is possible to investigate aqueous dispersions of these nanoparticles. Here, we prepared single bilayers of zwitterionic lipids on SiO2 nanoparticles ranging in size from 5 to 100 nm. It was possible to investigate both the CH stretching region, which has the most intense vibrational bands, as well as the weaker bands in the C-C skeletal region. Although the smaller nanoparticles with the highest surface area, and thus the most adsorbed lipid, were most easily observed, Raman spectra from the 100-nm SiO2 could also be obtained. The Raman spectra showed that the lateral packing of the lipids, and the relative number of trans conformers, increased as the nanoparticle size decreased. Furthermore, both the lateral packing and chain order were greater for the longer chain lipid, DSPC (18-carbon-atom alkyl chain) than for DPPC (16 C). These effects were attributed to increased interdigitation of the two halves of the bilayer with decreasing nanoparticle size. A band at 1122 cm-1, attributed to the presence of a terminal methyl group in a gauche orientation, was observed in all the SLBs. Formation of this kink was suggested to be another mechanism by which the alkyl chains increased their hydrophobic interactions, because with increasing curvature of the support there would otherwise be increasing free volume between the chains as the nanoparticle size decreased.
References
(1) C.S. Sweetenham and I. Notingher, Spectroscopy: An International Journal 24(1–2), 113–117 (2010).
(2) R.C. Spiker, Jr., and I.W. Levin, Biochim. Biophys. Acta, Lipids and Lipid Metabolism 388(3), 361–373 (1975).
(3) B.P. Gaber and W.L. Peticolas, Biochimica et Biophysica Acta, Biomembranes 465(2), 260–274 (1977).
(4) S. Ahmed and S.L. Wunder, Langmuir 25(6), 3682–3691 (2009).
(5) R.G. Snyder, S.L. Hsu, and S. Krimm, Spectrochimica Acta Part A - Molecular and Biomolecular Spectroscopy 34(4), 395–406 (1978).
(6) Y. Cho, M. Kobayashi, and H. Tadokoro, Journal of Chemical Physics 84(8), 4636–4642 (1986).
(7) S.L. Wunder, M.I. Bell, and G. Zerbi, Journal of Chemical Physics 85(7), 3827–3839 (1986).
(8) N. Yellin and I.W. Levin, Biochimica et Biophysica Acta 489(2), 177–190 (1977).
(9) R.G. Snyder, J.R. Scherer, and B.P. Gaber, Biochimica et Biophysica Acta 601(1), 47–53 (1980).
(10) L.T. Boni, S.R. Minchey, W.R. Perkins, P.L. Ahl, J.L. Slater, M.W. Tate, S.M. Gruner, and A.S. Janoff, Biochimica et Biophysica Acta 1146(2), 247–257 (1993).
(11) E.N. Lewis, R. Bittman, and I.W. Levin, Biochimica et Biophysica Acta 861(1), 44–52 (1986).
(12) R.J. Meier, A. Csiszar, and E. Klumpp, Journal of Physical Chemistry B 110(12), 5842–5844 (2006).
Selver Ahmed and Stephanie L. Wunder are with the Department of Chemistry, Temple University, Philadelphia, Pennsylvania.
Zhorro S. Nickolov is with Centralized Research Facilities, College of Engineering, Drexel University, Philadelphia, Pennsylvania.
New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.
