Rapid, Portable Pathogen Detection with Multiplexed SERS-based Nanosensors
Special Issues
A new application of surface-enhanced Raman spectroscopy (SERS) is described for quantifying low concentrations of pathogens with high reproducibility. In this novel assay, bacteria are captured and isolated using functionalized metal nanoparticles for rapid optical identification via SERS. Initial tests with a portable SERS system validated the ability to identify the presence of Escherichia coli and methicillin-resistant Staphylococcus aureus bacteria.
Antibiotic resistance rates are rising for many common pathogens, putting added pressure on healthcare providers to rapidly, and accurately, diagnose the cause of bacterial infections. New diagnostic methods must be sensitive, selective, and capable of multiplexed detection, as well as rapid, dependable, and portable enough to operate in a variety of settings. A new application of surface-enhanced Raman spectroscopy (SERS) shows great promise to meet these criteria. In this novel assay, bacteria are captured and isolated using functionalized metal nanoparticles for rapid optical identification via SERS. Initial tests with a portable SERS system validated the ability to identify the presence of Escherichia coli and methicillin-resistant Staphylococcus aureus bacteria, quantifying concentrations down to 10 colony-forming units per mL (cfu/mL), with high reproducibility. The system was also able to discriminate between the bacteria within the same sample matrix at clinically relevant concentrations (103cfu/mL), demonstrating its potential as a rapid, reliable on-site diagnostic for bacterial pathogens.
The proliferation of multidrug-resistant bacteria is a silent but growing crisis, acknowledged by public health authorities and regulatory agencies as an emergent global malady (1). Although resistance to antibiotic drugs may develop naturally via mutation, or by transfer of mobile genetic elements encoded with resistance genes, it is the selective pressure exerted through the widespread use, and misuse, of antimicrobial agents that causes these bacteria to selectively flourish, relative to weaker strains.
Both the absolute number and proportion of antimicrobial-resistant pathogens have increased this century. Although the prevalence of methicillin-resistant Staphylococcus aureus (commonly known as MRSA) is stabilizing or decreasing in Europe (2), infections due to antimicrobial-resistant Escherichia coli (E. coli) and Klebsiella pneumoniae (K. pneumoniae) are increasing across the continent. In the United States, Clostridium difficile, Enterobacteriaceae (including E. coli and K. pneumoniae), and Neisseria gonorrhoeae have been identified as the most urgent threats in a list of 18 drug-resistant pathogens (3). The rise of multi-drug resistance in E. coli is particularly concerning, as it is the most common Gram-negative pathogen in humans, causing a variety of maladies, ranging from general digestive upset to urinary tract infections and life-threatening bloodstream infections. Unlike MRSA, which is often acquired in a healthcare setting, most multidrug-resistant strains of E. coli originate in the general community. In one Icelandic study, increased prevalence of quinolone-resistant E. coli has even been traced back through the food chain to its presence in chicken feed (4).
As the costs to our healthcare systems grow, and the points of origin increase, so does the need for rapid, specific, and sensitive pathogen detection. Given that infections due to antimicrobial-resistant pathogens may stem from the food chain, the environment, or the clinic, detection methods must be flexible, and portable enough to be deployed in a wide variety of field conditions and by nonexperts. The vast majority of technologies currently used for the task fail on these counts, due to long analysis times (most approaches require culture prior to further analysis to increase bacteria concentration), the need for specialized equipment or staff, high cost, or additional sample preparation steps; these include staining, culturing and biochemical assays, real-time polymerase chain reaction (RT-PCR), and microarray assays like enzyme-linked immunosorbent assay (ELISA). Lab-on-a-chip technology seeks to streamline sample handling and concentration steps to reduce human error, and improve accuracy in a portable format, but the technology still lacks the simplicity of our technique.
SERS for Rapid Pathogen Detection
Surface enhanced Raman spectroscopy (SERS) offers a rapid and portable alternative to the existing methods for bacterial detection. SERS leverages the specificity of Raman signals with their sharp, well-defined peaks to provide a fingerprint unique to each analyte, with enhanced sensitivity due to interaction with silver or gold nanostructures. When in close proximity, surface plasmon resonance in the noble metal generates an amplified electromagnetic field that enhances the Raman signal. If the laser frequency used is tuned to the absorbance maxima of the analyte, additional enhancement can be observed, an effect known as surface enhanced resonance Raman scattering (SERRS). The combined enhancement can yield up to ~1014 in total signal amplification. Using silver-coated gold nanorods, SERRS has been used by Wang and associates to differentiate between strains of carbapenem-resistant E. coli and carbapenem-sensitive E. coli with almost 100% accuracy, using orthogonal partial least squares (OPLS) discriminant analysis of their spectra (5).
SERS using functionalized nanoparticles (NPs) tagged with resonant Raman reporter molecules has proven ideal for multiplexed detection of bacteria, offering excellent selectivity, high photostability, and lack of spectral crosstalk. The addition of a "magnetic separation" step to isolate bacteria from the host matrix has been shown to improve detection limits to within clinically relevant levels (103 colony-forming units [cfu]). This approach enabled Guven and colleagues to report detection limits of eight cfu/mL in water for E. coli (6), and Najafi and associates 102 cfu/mL in apple juice (7). Wang and colleagues were able to simultaneously detect Salmonella enterica and S. aureus in spiked spinach solution and peanut butter at concentrations of 103 cfu/mL, demonstrating the potential of the approach in complex matrices (8). Although these studies meet the threshold for speed and minimum detection limit, they leave opportunities for further improvement in sensitivity and ease of sample preparation.
A Novel SERS Assay
To this end, we developed a highly sensitive, efficient, and easy-to-use sandwich SERS assay for the isolation and multiplexed detection of bacteria (Figure 1). Lectin- functionalized magnetic nanoparticles (MNPs) are used to capture and isolate bacteria within the sample, while SERS-active silver nanoparticles (AgNPs), functionalized with bacteria-specific antibodies and Raman reporter molecules, bind to each specific strain for interrogation.
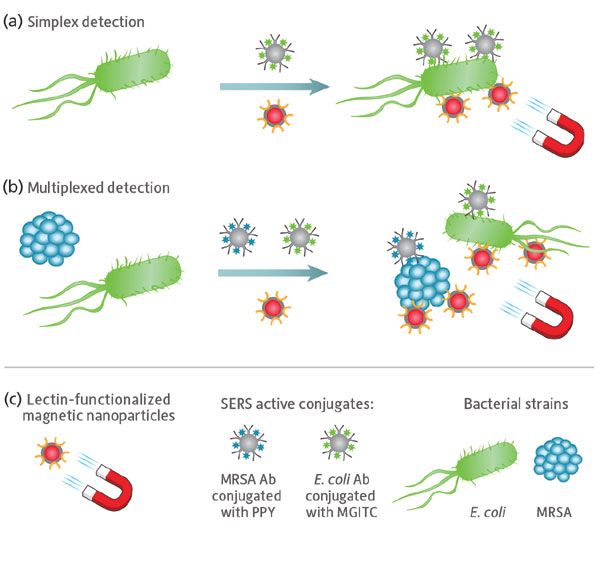
Figure 1: Schematic illustrating the sandwich SERS assay used for: (a) single-pathogen and (b) multiplexed pathogen detection. (c) Lectin-functionalized silver-coated magnetic nanoparticles facilitate magnetic separation of all bacterial strains from the sample matrix, while SERS active conjugates composed of silver nanoparticles functionalized with strain-specific antibodies and unique Raman reporter molecules facilitate optical detection of each pathogen via SERS.
Lectins were chosen as the functional group for the magnetic nanoparticles, because of their affinity for the sugars expressed on the surface of bacteria; the MNPs then act as a handle by which magnetic separation can be used to capture bacteria indiscriminately from the sample matrix. This approach allows any unbound material to be washed away, concentrating the sample for increased signal, less background, and elimination of false positives.
SERS-active conjugates facilitate optical detection of the bacterial pathogens. Each conjugate type consists of silver nanoparticles functionalized with strain-specific bacterial antibodies and a unique Raman reporter molecule, providing each bacterial strain under interrogation with a bespoke optical fingerprint when investigated using SERS. The small size of the AgNPs, relative to the bacteria surface, permits multiple binding sites, allowing the AgNPs to come in close proximity to one another and form SERS hot spots for increased Raman signal. Unbound AgNPs are washed away during the concentration stage, further benefiting signal to noise of the detection technique.
The use of lectin-functionalized MNPs makes this separation step novel and efficient in capturing and isolating bacteria from a sample matrix, while concurrent addition of bacteria-specific SERS conjugates simplifies and speeds sample preparation. Previous work by our group has shown this detection assay to be effective in isolating and detecting E. coli, S. typhimurium, and MRSA down to concentrations of 101 cfu/mL individually, using a benchtop Raman microscope system for SERS detection. We have also demonstrated triplex detection, successfully identifying each bacterial strain within the same sample matrix (9). The present study establishes transferability of the technique to a more compact, cost-effective, and portable system, without compromise to detection limit or multiplexing ability, greatly expanding its potential for practical use.
Experimental
Synthesis of silver nanoparticles and silver coated magnetic nanoparticles, and the preparation of the biomolecule-AgNP conjugates that were used in these studies have previously been reported by our group (9). Detailed protocols for the preparation and characterization of these colloidal suspensions are provided in the supporting information (ESI) of this paper, and previously published in the journal Analytical Chemistry in December of 2017.
Bacterial Strains
E. coli ATCC 25922 and S.aureus (methicillin resistant) ATCC BAA-1766 were used in this study. E. coli and MRSA were grown on Luria-Bertani (LB) Miller agar in an aerobic atmosphere for 24 h at 37 oC. Harvested cells were suspended in LB broth to obtain an optical (OD600 nm) of 0.6. Cells were then plated onto LB agar plates as triplicates, and incubated for 24 hours, as described previously. All the strains were grown using the same batch of culturing plates to reduce any potential unwanted phenotypic variation.
Bacterial Sample Preparation
Bacterial slurries were prepared by harvesting the biomass from the surface of each plate using sterile inoculating loops, and resuspending in physiological saline solution (1 mL; 0.9% NaCl). The prepared bacterial slurries were washed by centrifugation (E. coli, 650 g for 5 min; MRSA, 1600 g for 5 min), and the pellets resuspended in 1 mL saline. The wash step was repeated a further twice with the final re-suspension being in deionized water (1 mL; d.H2O). Note the OD600 nm was recorded for all samples, and bacterial concentrations were obtained by serial dilutions based on plate-counting results. All samples were stored at -80 °C until further analysis.
Detection Assay
The novel method for bacterial detection described previously by Kearns and colleagues (9) was modified slightly for use in this study.
Single Pathogen Detection
For each of the bacterial pathogens, the following procedure was carried out: SERS active antibody functionalized silver nanoparticles (200 µL; Ab-AgNP) were added together with lectin functionalized silver coated magnetic nanoparticles (200 µL; Con A-Ag@MNP), and a specific bacterial strain (50 µL, 104 cfu/mL). Note for the control sample, the bacteria was replaced with d.H2O (50 µL) only. The sample was mixed thoroughly for 30 min before being placed in a magnetic rack for a further 30 min to allow the sample to collect. The clear supernatant was removed and the sample re-suspended in d.H2O (600 µL) ready for analysis. The same procedure was followed for the concentration study of E. coli, except the bacteria concentration was varied from 105 to 101 cfu/mL. It should be noted that each sample was prepared in triplicate.
Multiple Pathogen Detection
The two sets of antibody functionalized silver nanoparticles (100 µL of each conjugate) were added together with lectin-functionalized silver-coated magnetic nanoparticles (200 µL); plus the two bacterial strains (50 µL of each pathogen; 103 cfu/mL). Note for the control sample, the bacteria was replaced with d.H2O (100 µL) only. The same procedure as described above (for the single pathogen detection) was employed.
SERS and Multivariate Analysis
Raman reporters used were malachite green isothiocyanate (MGITC) and 4-(1H-pyrazol-4-yl)-pyridine (PPY) and these were used to detect E. coli and MRSA, respectively.
The samples were analyzed immediately after preparation using a WP 532 compact Raman spectrometer (50 µm slit, temperature-regulated detector set at 15 °C) and fiber optic probe (Wasatch Photonics, Morrisville, North Carolina), with 532 nm laser excitation (InPhotonics, Norwood, Massachusetts). The probe was focused directly into a 1 mL glass vial containing 600 µL of the bacteria–NP conjugate solution. All the measurements had a 1 s acquisition time, and a laser power operating at 7.7 mW. For all Raman spectra, data handling was carried out in Excel software, and no preprocessing was required. The peak intensities were obtained by scanning three replicate samples five times, and in all plots the error bars represent one standard deviation. The peak heights were calculated by averaging the peak intensities acquired from the 15 scans, and then subtracting the maximum intensity at 1620 cm-1 (E. coli) and 960 cm-1 (MRSA) from the base of the peak. Following this, the signal enhancements were calculated by dividing the peak heights of the sample by the peak height of the control. Furthermore, the relative standard deviations were calculated (% RSD) by dividing the average standard deviation by the mean peak intensity, and multiplying by 100.
All multivariate statistical analysis was carried out in MATLAB software (The MathWorks, Natick, Massachusetts). Prior to conducting principal component analysis (PCA), the data was scaled. PCA was performed on three different data sets, two consisting of spectra obtained from single pathogen detection experiments, and one data set obtained for the multiplex detection of the two pathogens (10).
Results and Discussion
Validation of Single Pathogen Detection
To validate the SERS assay for simplex detection of each individual bacterial pathogen, we compared spectra of bacterial samples treated with the E. coli assay (SERS active conjugates + MNPs) to control samples. The total analysis time was ~1 h. The distinct Raman spectrum seen for 104 cfu/mL E. coli samples treated with the assay is due to the Raman reporter MGITC (selected for use with E. coli antibodies), and thus confirms the presence of E. coli in the sample (Figure 2a). In contrast, the control (water + assay) showed only very weak signal attributable to unbound residual SERS conjugates not washed away during the magnetic separation step. Comparing the characteristic MGITC peak at 1620 cm-1, a signal enhancement of 14.5 times was observed for the E. coli bacteria over the control (Figure 2b), matching the 10 times enhancement observed previously using a Raman microscope for detection (9).
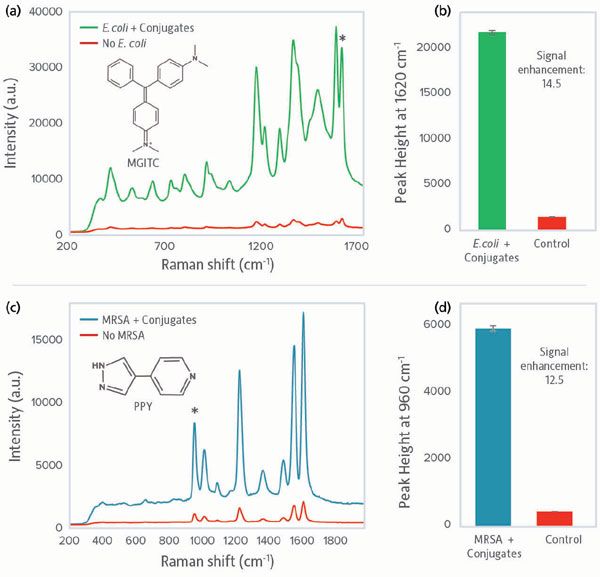
Figure 2: SERS spectra obtained from single pathogen detection using the sandwich SERS assay. (a) SERS spectrum of MGITC observed when detecting E. coli (green) and the control spectrum representing when no bacteria was present (red); (b) SERS peak intensities at 1620 cm-1 for assay and control when detecting E. coli; (c) SERS spectrum of PPY observed when detecting MRSA (blue) and the control spectrum (red); (d) SERS peak intensities at 960 cm-1 for assay and control when detecting MRSA. Raman intensity is given in arbitrary units (a.u.).
Similarly, 104 cfu/mL MRSA samples treated with the MRSA assay containing the Raman reporter PPY clearly confirmed presence of MRSA in the sample versus the control (Figure 2c). Examining the characteristic PPY peak at 960 cm-1, a signal enhancement of 12.5 times was observed for the MRSA bacteria over the control (Figure 2d), matching the 11 times enhancement observed previously using a Raman microscope. Transfer of the method to detection using a portable Raman system did not compromise the level of discrimination which could be achieved, and may have increased it somewhat. We believe the sampling geometry with the portable system may be more conducive to this type of assay, because it accessed a larger optical area and therefore more conjugates. Further work will directly compare the two detection methods using the same sample set.
Limit of Detection, Single Pathogen
Having established the ability of our portable bionanosensor system to clearly identify pathogens within a matrix, we characterized the detection limit for E. coli using a concentration series from 105 to 101 cfu/mL. Spectra for concentrations up to 104 cfu/mL showed a monotonic increase in signal, with clear discrimination at all concentrations versus the control (Figure 3a). Comparing signal intensity for the characteristic MGITC peak at 1620 cm-1, E. coli could be clearly detected down to 101 cfu/mL, at which concentration a signal enhancement of 3 times over the control was observed (Figure 3b). Above 104 cfu/mL, the signal decreased; we believe it was dampened due to self-absorbance of Raman light when large numbers of conjugated bacteria are present. If a high concentration of bacteria is present, the sample could be diluted and reanalyzed for accurate signal response.
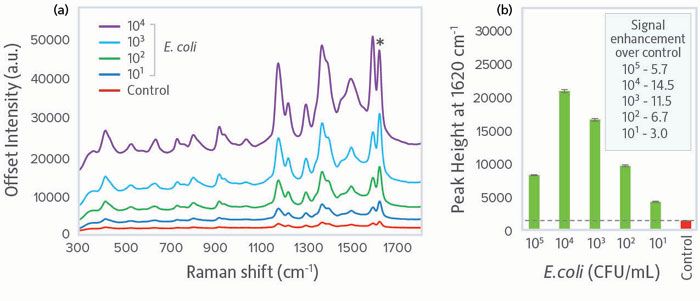
Figure 3: Concentration series for E. coli showing: (a) Comparison of SERS spectra for concentrations 101-104 cfu/mL versus the control, and (b) peak intensity at 1620 cm-1 as a function of concentration. The dashed line gives visual clarification of the SERS peak intensity of the control. Raman intensity is given in arbitrary units (a.u.).
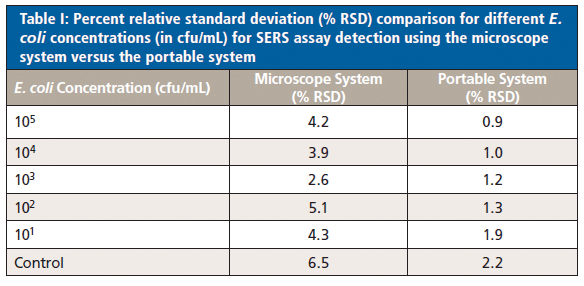
Comparing the relative standard deviation (% RSD) of the portable system to results from the earlier microscope system study, the portable system showed much lower variance at all concentrations (Table I). This result indicates improvement in reproducibility, which may be due to the larger sampling area and increased laser power, and should be explored in further work.
Multiplex Detection
For a bionanosensor to be of practical use in healthcare, food safety, or environmental testing, it must be capable of multiplexed pathogen detection, both Gram-negative and Gram-positive. To this end, we tested the performance of our assay when both E. coli (Gram-negative) and MRSA (Gram-positive) were present at a clinically relevant concentration, 103 cfu/mL. SERS conjugates for both pathogens were included in the assay. The resulting duplex sample spectrum shared features of both the E. coli and MRSA reference spectra, and clear discrimination versus the control (Figure 4a). Given that the peak previously used to identify E. coli overlaps a SERS peak for the MRSA Raman reporter (1620 cm-1, shown with a green star), we instead used the peak at 1180 cm-1 to confirm the presence of E. coli. Both peaks were clearly present in the duplex sample. Furthermore, comparison of the duplex sample to the control using the 1620 cm-1 peak present in both Raman reporters yielded a signal enhancement of 8.4 times, clearly demonstrating a unique biorecognition interaction between the SERS conjugates and the two bacterial strains (Figure 4b).
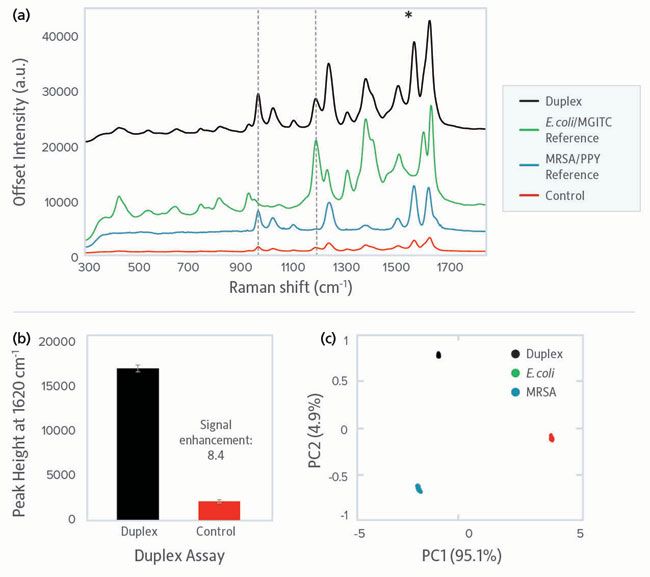
Figure 4: Duplex pathogen detection using the SERS assay for E. coli and MRSA at 103 cfu/mL. (a) Stacked SERS spectra showing the spectra obtained from the detection of both bacterial pathogens simultaneously (black) versus each pathogen separately and a control sample (red). MGITC spectrum (green) represents E. coli and PPY spectrum (blue) represents MRSA. The black dotted lines show peaks unique to each Raman reporter, used to identify the presence of the bacterial targets. (b) Comparative peak intensities at 1620 cm-1 for assay and control. (c) PCA scores plot showing the relationship between the multiplex spectra (black) and the E. coli (green) and MRSA (blue) single pathogen spectra. Raman intensity is given in arbitrary units (a.u.).
To confirm detection of each pathogen, principal component analysis (PCA) was performed on the multiplex spectrum and two single pathogen spectra (Figure 4c). The tight clustering observed for the fifteen scans taken for each individual pathogen, as well as the duplex sample, shows the excellent reproducibility and discrimination in the identification of pathogens that is possible with a portable system.
Conclusion
A compact SERS system providing all the requirements for a field-deployable assay (sensitivity, specificity, reproducibility, cost, portability, ease of use, and speed of analysis) has been demonstrated. This novel approach employs a sandwich-type nanoparticle assay in which magnetic separation is used to capture SERS active bacteria complexes for isolation and concentration from the sample matrix. Using this detection assay, cell concentrations as low as 10 cfu/mL were readily detected in single pathogen tests for E. coli and MRSA using a compact, portable Raman detection system. Duplex detection clearly and simultaneously identified the presence of both pathogens, with discrimination being made using both PCA and SERS analysis.
The ability of this portable bionanosensor to detect bacterial concentrations below the requirements for clinical diagnosis, and to discriminate between bacterial pathogens within a duplex system in ~1 h with minimal sample preparation, demonstrates its potential as a field-deployable technique for use in healthcare, food, and environmental testing. As sample preparation time and system size are further reduced, its potential to provide rapid, sensitive detection in a field-deployable format will surely play a role in global efforts to curb the spread of antimicrobial-resistant pathogens.
References
(1) I. Roca, M. Akova, F. Baquero, J. Carlet, M. Cavaleri, S. Coenen, J. Cohen, D. Findlay, I. Gyssens, O.E. Heure, G. Kahlmeter, H. Kruse, R. Laxminarayan, E. Liébana, L. López-Cerero, A. MacGowan, M. Martins, J. Rodríguez-Bañ o, J.-M. Rolain, C. Segovia, B. Sigauque, E. Taconelli, E. Wellington, and J. Vila, New Microbes New Infect. 6, 22-29 (2015).
(2) European Antimicrobial Resistance Surveillance Network, Surveillance of Antimicrobial Resistance in Europe (European Centre for Disease Prevention and Control, Solna, Sweden, 2017).
(3) Centers for Disease Control and Prevention, Antibiotic Resistance Threats in the United States (United States Department of Health and Human Services, Washington, D.C.,2013).
(4) T.R. Thorsteinsdottir, G. Haraldsson, V. Fridriksdottir, K.G. Kristinsson, and E. Gunnarsson, Emerg Infect Dis. 16, 133–135 (2010).
(5) J. Li, C. Wang, H. Kang, L. Shao, L. Hu, R. Xiao, S. Wang, and B. Gu, RSC Adv. 8, 4761–4765 (2018).
(6) B. Guven, N. Basaran-Akgul, E. Temur, U. Tamer, and I.H. Boyaci, Analyst 136, 740-748 (2011).
(7) R. Najafi, S. Mukherjee, J. Hudson, A. Sharma, and P. Banerjee, Int. J. Food Microbiol. 189, 89-97 (2014).
(8) Y. Wang, S. Ravindranath, and J. Irudayaraj, Anal. Bioanal. Chem. 399, 1271-1278 (2011).
(9) H. Kearns, R. Goodacre, L.E. Jamieson, D. Graham, K. Faulds, Anal. Chem. 89(23), 12666-12673 (2017).
(10) D.H. Kim, R.M. Jarvis, Y. Xu, A.W. Oliver, J.W. Allwood, L. Hampson, I. Hampson, and R. Goodacre, Analyst 135, 1235-44 (2010).
Hayleigh Kearns, Lauren E. Jamieson, Duncan Graham, and Karen Faulds are with the Department of Pure and Applied Chemistry at the University of Strathclyde in Strathclyde, United Kingdom. Cicely Rathmell is the Vice President of Marketing at Wasatch Photonics in Morrisville, North Carolina. Direct correspondence to: marketing@wastachphotonics.com
Advanced Raman Spectroscopy Method Boosts Precision in Drug Component Detection
April 7th 2025Researchers in China have developed a rapid, non-destructive Raman spectroscopy method that accurately detects active components in complex drug formulations by combining advanced algorithms to eliminate noise and fluorescence interference.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.
New Multi-Spectroscopic System Enhances Cultural Heritage Analysis
April 2nd 2025A new study published in Talanta introduces SYSPECTRAL, a portable multi-spectroscopic system that can conduct non-invasive, in situ chemical analysis of cultural heritage materials by integrating LIBS, LIF, Raman, and reflectance spectroscopy into a single compact device.
