Article
Special Issues
Spectroscopy Supplements
Simple and Effective Control of Spectral Overlap Interferences in ICP-MS
Author(s):
The authors discuss a new approach to the control of spectral overlap interferences in inductively coupled plasma–mass spectrometry.
A new approach to the control of spectral overlap interferences in inductively coupled plasma–mass spectrometry (ICP-MS) is described. In this technique, called the collision reaction interface (CRI), gas is injected directly into the sampled plasma as it passes through the tips of the ICP-MS interface cones. The introduced gas collides and reacts with potentially interfering ions, removing them from the plasma before any ions are extracted by the ion optics. The capabilities of the CRI technique are illustrated with reference to the determination of Se under "hot plasma" conditions, of 52Cr in the presence of 2-propanol under "hot plasma" conditions, and of a range of trace metals in certified urine samples, with no matrix matching.
Some common interferences in inductively coupled plasma–mass spectrometry (ICP-MS) are caused by ions having the same mass-to-charge ratio (m/z) as the isotope ions of interest (1). The most common techniques for controlling these interferences involve either the use of "cool plasma" conditions (2) or of various pressurized multipole ion guides (3), in which the ion beam is exposed to a gas after it has been extracted from the plasma by the ion optics. As shown in Figure 1, the collision reaction interface (CRI) (4) works by injecting a gas into the plasma as it passes through the tip of one or both of the interface cones.

Figure 1
Collisions between gas molecules and plasma ions change the kinetic energy of the ions and can induce reactions such as charge transfer, proton transfer, collisional excitation–dissociation, electron–ion reaction, and ion–molecule reaction. Ions that normally would cause spectral interferences are more susceptible to such processes than are the analyte ions. Consequently, interfering ions can be selectively removed from the plasma before any ions are extracted into the ion optics. The high plasma density in the interface apertures leads to a high collision frequency. Hydrogen and helium are used as CRI gases; they provide efficient interference attenuation, and there is no need to use expensive, toxic, and corrosive gases such as ammonia. Cleaning the CRI is as simple as the routine cleaning of the interface cones in a conventional ICP-MS instrument.
Examples of the Removal of Interfering Ions with the Collision Reaction Interface
Use of Hydrogen to Remove Ar2+
The argon dimer ion Ar2+ is always present under "hot plasma" conditions. It interferes with the determination of selenium, because the three most abundant isotopes of Se have the same nominal mass as Ar2+ ions. Specifically, 40Ar2+ overlaps with 80Se+, 38Ar40Ar+ overlaps with 78Se+, and 36Ar40Ar+ overlaps with 76Se+. The formation of Ar2+ is suppressed under "cool plasma" conditions (2), but these are not useful for Se determinations because of the high ionization energy of this element (941 kJ/mol, 9.75 eV). When H2 is injected into the sampled plasma through the CRI, Ar2+ ions collide with H2 molecules. A chemical reaction then occurs, in which a proton is transferred from a H2 molecule to an Ar2+ ion, forming ArH+, a neutral H atom and a neutral Ar atom. The product ion ArH+ then collides with another H2 molecule and a proton is transferred from the ion to the molecule, forming a neutral Ar atom and an H3+ ion. (5) The final product ion H3+ (m/z = 3 amu/unit electronic charge) does not interfere with any isotopes of interest in ICP-MS, and in any case is likely to be destroyed by reaction with plasma electrons to form H2 and a neutral H atom.
Figure 2 shows a time scan graph for 115In+ and 80Se+, where the CRI gas flow is increasing stepwise. Because no Se was present in the test solution, the measured signal for 80Se+ is due entirely to the interfering ion 40Ar2+. Also shown in Figure 2 is the signal ratio of 115In+/80Se+. The time scan was carried out with a test solution (Var-IS-1) containing 10 μg/L of internal standard elements (Bi, In, Li, Sc, Tb, and Y). During the time scan, the H2 gas was injected into the plasma through the CRI skimmer cone tip. The H2 flow rate was stepped from 0, 20, 50, 80, 100, to 120 mL/min with about 50 s between each step.

Figure 2
The efficiency of interference reduction–removal using CRI is demonstrated clearly in Figure 2, in which the signal for the interfering species Ar2+ (scanned as 80Se+) is decreasing progressively with increasing H2 gas flow rate, while the signal to interferent level (indicated by the ratio 115In+/80Se+) is improving continuously. As seen in Figure 2, at a H2 flow rate of around 120 mL/min, the interference from Ar2+ has been removed completely, and the sensitivity for the analyte 115In+ is still maintained at a high level (over 50,000 c/s per 1 μg/L of indium).
Use of Helium to Remove ArC+
The argon carbide ion 40Ar12C+ interferes with the major isotope of chromium (52Cr, natural abundance 83.79%). When He is injected into the sampled plasma through the CRI, interaction between the electron clouds of the He atoms and those of polyatomic ions can make a large polyatomic interfering ion such as ArC+ rotationally and vibrationally excited. In subsequent collisions, an excited ArC+ ion can receive sufficient energy to bring about its dissociation, removing the 40Ar12C+ interference from the determination of 52Cr.
Figure 3 shows interference reductions in three-dimensional graphs, where the signals for 52Cr+ and the ratio 59Co+/52Cr+ were measured at various CRI gas flow rates. The test solution contained 10 μg/L Co in a matrix solution of 1% (v/v) concentrated HNO3, 1% (v/v) concentrated HCl and 1% (v/v) 2-propanol. Helium was used as the CRI gas. Because no Cr was present in the test solution, the 52Cr+ signal is due to interfering polyatomic ions such as 40Ar12C+, 38Ar14 N+, and 35Cl16O1H+. As shown in Figure 3a, this signal for interfering polyatomic ions decreased with increasing He flow at the tip of the skimmer cone. With He flows over 120 mL/min, the signal for interfering ions was reduced to a minimum. In contrast, the He flow at the tip of the sampler cone had little effect. The efficiency of interference reduction is demonstrated in Figure 3b, where the signal ratio of 59Co+/52Cr+ (that is, the ratio of the signal from a real analyte to that from interfering ions) improved with increasing He flow rate at the skimmer. Again, the He flow at the sampler had no significant effect.

Figure 3
The results in Figure 3 are typical of experimental results obtained so far, which all show that gas injected into the sampler cone is much less effective at reducing or removing interfering ions. This is not surprising, because the concentration of plasma ions in the sampler is about 1000 times less than in the skimmer. It is therefore possible to obtain a much larger ratio of CRI gas to plasma ions at the skimmer than at the sampler for the same gas flow. In Figure 3, the maximum gas flow into the sampler was 10 times that into the skimmer; use of greater gas flows was considered impractical.
Typical Detection Limits in the CRI Mode
Table I lists typical detection limits (DL) measured in the CRI mode under "hot plasma" conditions, determined as the concentration corresponding to three times the standard deviation of 10 replicates of a blank solution (1% [v/v] concentrated HNO3). These measurements were made under routine analytical laboratory conditions, not "clean-room" conditions.

Table I: Typical detection limits for selected isotopes
Long-Term Stability in the CRI Mode
Figure 4 shows the long-term signal stability in a high total dissolved solids matrix in the CRI mode over a period of 5 h, using a solution containing 0.1% (w/v) (1000 mg/L) NaCl, spiked with 1 μg/L of various analytes. The results were extremely stable over 5 h. The relative standard deviations (RSD) of the measured signals for most analytes did not exceed 5% over the 5 h. Similar results also were obtained using He as the CRI gas.
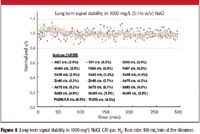
Figure 4
An Application of the CRI: Determination of Trace Metals in Urine
Experimental
Instrument and Operating Conditions
The work was carried out on a Varian 820-MS ICP-MS system (Varian, Palo Alto, California), which includes the CRI. Table II shows conditions for the urine analysis.
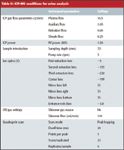
Table II: ICP-MS conditions for urine analysis
Materials and Reagents
As an example of the use of the CRI in a real analysis, we analyzed two certified urine samples for a range of trace metals, using calibration solutions prepared in dilute nitric acid, without matrix matching.
Sample Treatment
Urine metal control materials, UMC L-1 and UMC L-2, were reconstituted according to the procedure supplied with the materials. For trace metals determination with the ICP-MS system, the reconstituted urine solution was further diluted by a factor of 10, by adding 1 mL of the urine into a precleaned tube containing 9 mL of 2% (v/v) concentrated HNO3.
Sample Analysis
Diluted (1:10) urine solution was used for direct ICP-MS analysis. Calibration solutions were prepared in 2% (v/v) concentrated HNO3, with no matrix matching.
High-purity nitric acid and hydrochloric acid (both AR Select Plus, Mallinckrodt Baker, Phillipsburg, New Jersey), 2-propanol (SupraSolv for gas chromatography, Merck KGaA, Darmstadt, Germany), and deionized water (18 MΩ cm, Millipore Milli-Q, Billerica, Massachusetts) were used in the preparation of sample and calibration solutions. All labware, new or used, was cleaned thoroughly by acid washing and rinsing, and the clean containers were left filled with 2% (v/v) concentrated HNO3 until use. Three multielement calibration solutions were prepared for the urine analysis, at concentration levels of 1 μg/L, 5 μg/L, and 10 μg/L, by diluting a 10-mg/L multielement stock solution (SPEX CertiPrep, Inc., Metuchen, New Jersey) with 2% (v/v) concentrated HNO3. An internal standard solution, containing 20 μg/L of 6Li, 45Sc, 115In, 89Y, 159Tb, and 209Bi, was prepared by diluting a 100-mg/L internal standard stock solution (Var-IS-1, Inorganic Ventures, Inc., Lakewood, New Jersey) with 2% (v/v) concentrated HNO3. The diluted internal standard solution was added to the nebulizer through a "Y-piece" using the third channel of the ICP-MS system sampling pump. Two urine metals control materials (Lyphochek urine metals control, Level-1 (LOT 69101) and Level-2 (LOT 69102), Bio-Rad Laboratories, Irvine, California) were then selected as the sample for the direct trace element determination in urine.
Results and Discussion
The analyte concentrations measured in two certified urine metal-control materials using the ICP-MS system are listed in Table III. The measured values shown in Table III are the average of two repeat measurements.
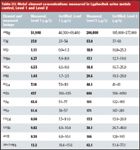
Table III: Metal element concentrations measured in Lyphochek urine metals control, Level 1 and Level 2
These results show excellent agreement between measured and certified values. The measured concentrations for Ni in UMC L-1 and Se in UMC L-2 are slightly lower than the certified range. This difference is likely to be due to the different techniques used for the analysis, because the certified values for both Ni and Se were obtained using atomic absorption spectroscopy rather than ICP-MS.
Summary and Conclusion
The collision reaction interface provides a simple, but very effective, approach to removing common interferences in ICP-MS analysis. This unique approach reduces spectral overlap interferences from common polyatomic ions on elements such as As, Se, Cr, V, and Fe, thus achieving lower detection limits for these elements under "hot plasma" conditions, even for samples (such as urine) that have complex matrices. The CRI does not pass the extracted ion beam through a pressurized multipole; instead, gases are injected directly into the plasma through the tips of the interface cones to remove interfering ions by collisions or chemical reactions. This simple and innovative approach reduces or removes interfering ions before any ions are extracted from the sampled plasma into the ion optics. The CRI approach also provides operational advantages including low maintenance; easy operation; and no use of toxic, corrosive, or expensive gases such as ammonia.
Iouri Kalinitchenko, XueDong Wang, and Barry Sturman are with Varian, Inc., Palo Alto, California.
References
(1) R.S. Houk, Anal. Chem. 58, 97A–105A (1986).
(2) S.D. Tanner, J. Anal. At. Spectrom. 10, 905–921 (1995).
(3) S.D. Tanner, V.I. Baranov, and D.R. Bandura, Spectrochim. Acta, Part B 57B, 1361–1452 (2002).
(4) I. Kalinitchenko, United States Patent 7,329,863 (2008).
(5) V.G. Anicich, An index of the literature for bimolecular gas phase cation-molecule reaction kinetics, JPL Publication 03-19, National Aeronautics and Space Administration, Jet Propulsion Laboratory, California Institute of Technology, Pasadena, 2003, pp. 506–510.

Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.





