Simultaneous Determination of 50 Elements in Geological Samples by ICP-MS Combined with ICP-OES
A method combining inductively coupled plasma–mass spectrometry (ICP-MS) with inductively coupled plasma–optical emission spectrometry (ICP-OES) was developed for multielement determination of 50 species of major, minor, micro, and trace, rare earth, and rare elements in geological samples. The geological samples of stream sediment, soil, and rock were dissolved with HCl-HNO3-HF-HClO4. The instrumental operating parameters were optimized, and the best analytical parameters were determined. The influences of ICP-OES and ICP-MS interferences were investigated. The optimal spectral lines and best isotopes were chosen, and the internal standard elements of 185Re and 103Rh were selected to compensate for signals drifting and for matrix effects. Therefore, the standard solutions were prepared by simultaneously dissolving the certified reference material (CRM) with the samples. The elements of Ti, V, Mn, Ba, Sr, Li, Be, Sc, Co, Ni, Cu, Zn, and Pb were determined by both ICP-MS and ICP-OES, which show a good correlation between measured values. The methods were used to determine the CRM of stream sediment (GBW07301a, GBW07365), soil (GBW07408, GBW07452) and rock (GBW07107, GBW07121 and GBW07122), and the determined results were in agreement with the certified values for simultaneous determination of 50 elements for bulk geological samples.
Rare earth elements (REEs) and rare elements (REs) have irreplaceable functions and broad application prospects in high-tech fields such as electronic, energy, aerospace, military, medicine, and so on. They are also important for geological prospecting because of their unique properties. Therefore, accurately determining contents of major, minor, trace elements, REEs and REs in geological samples are of significance to the administration, evaluation, and comprehensive utilization of mineral resources.
In recent years, the analytical methods for REEs, REs, and other major, minor, micro, and trace elements in geological samples mainly include X-ray fluorescence (XRF) (1,2), atomic absorption spectroscopy (AAS) (3,4), atomic fluorescence spectroscopy (AFS) (5,6), instrumental neutron activation analysis (INAA) (7), inductively coupled plasma–optical emission spectroscopy (ICP-OES) (8,9), and inductively coupled plasma–mass spectrometry (ICP-MS) (10,11). Compared with AAS, AFS, and other methods, ICP-OES has the advantage of small self-absorption effects, low background noise, and simultaneous determination of multi-elements. ICP-OES can also help determine major and minor elements (8,12). Nevertheless, ICP-OES has its own limitations in the determination of micro and trace elements, where the accuracy of the analytical results could be affected by the complexity of the separation and enrichment process. ICP-MS is the most commonly used method for the simultaneous determination of multi-elements, and the technique is superior to ICP-OES because of its detection limits, precision, and sensitivity, and it also can be used to determine REEs, REs, and other trace elements (10,13). However, limitations of ICP-MS include the interference from matrix effects and polyatomic ions (10,14). Thus, it is difficult to obtain ideal results by a single analytical method because the major, minor, micro, and trace elements in geological samples have a large range of concentration. As a result, combining different analytical techniques is an effective method for the simultaneous determination of multi-elements in geological samples (7,15).
In this study, an improved method using the HCl-HNO3-HF-HClO4 system to dissolve the geological samples, and the concentrations of 50 species of major, minor, microtrace, REEs, REs, and other trace elements, were simultaneously determined using both ICP-OES and ICP-MS. These methods were applied to analyze the certified reference material of stream sediment, soil, and rock. The results were in good agreement with the certified values.
Experimental
Instruments and Operation Conditions
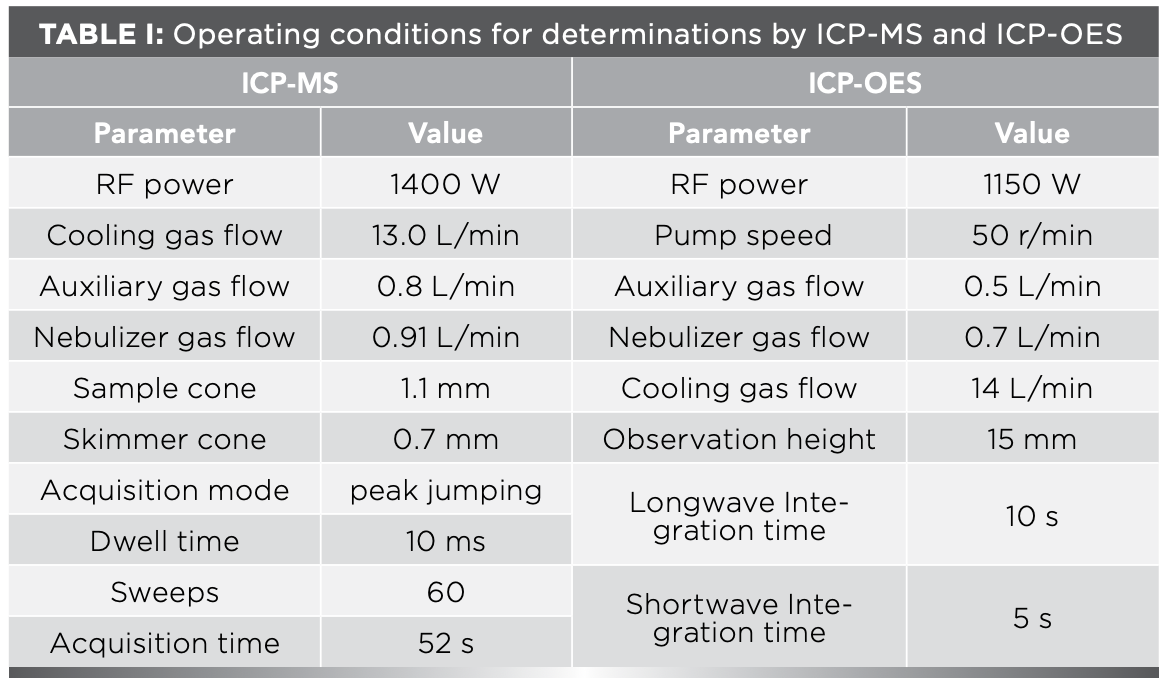
The experiments were made using a quadrupole ICP-MS X series II (Thermo Fisher Scientific) and a iCAP6300 ICP-OES instrument (Thermo Fisher Scientific). Details of the instrument operating conditions and parameters are summarized in Table I.
Reagents and Materials
Guaranteed reagent acids of HCl, HNO3, HF, and HClO4 were used in geological sample digestion and the preparation of the solution. High-purity water from a MilliQ-system was used throughout the ICP-MS and ICP-OES procedures.
The test samples were certified reference material of stream sediment (GBW07301a, GBW07309, GBW07365), soil (GBW07408, GBW07452, GBW07457), and rock (GBW07104, GBW07107, GBW07121, GBW07122). They were developed by the Institute of Geophysical and Geochemical Exploration (IGGE) of the Chinese Academy of Geological Sciences (CAGS).
Sample Preparation
The procedures used for sample decomposition are as follows: the sample powders were dried at 105 oC for 2 h prior to digestion, and 0.2500±0.0005 g of sample was accurately weighed and placed into a 50 mL polytetrafluoroethylene (PTFE) beaker. After wetted with a few drops of high-purity water, 5 mL of HCl was added, and the beaker was heated with an electric hot plate at 200 ℃ and evaporated to incipient dryness. Then, 5 mL of HNO3, 10 mL of HF, and 2 mL of HClO4 were added and the temperature raised to 220 ℃. After the white acid mist had run out, the temperature dropped to 180 ℃ and 10 mL aqua regia was added immediately. When the tiny bubbles were exhausted, 5 mL high-purity water was added. After cooling, the solution was transferred into a clean PTFEcolorimetric tube with high-purity water and finally diluted to 25 mL.
The sample solution was divided into two parts; one was determined for 20 elements of Al, Ba, Be, Ca, Co, Cu, Fe, K, Li, Mg, Mn, Na, Ni, P, Pb, Sc, Sr, Ti, V, and Zn by ICP-OES, and the other was diluted 10 times with 2% HNO3, Rh, and Re added as internal standards, with the concentrations of Rh and Re in the solution at 10 ng/mL (ppb). Afterwards, 42 elements of Li, Be, Sc, Ti, V, Mn, Co, Ni, Cu, Zn, Ga, Rb, Sr, Y, Nb, Mo, Cd, In, Cs, Ba, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Tl, Pb, Bi, Th, and U were determined by the ICP-MS instrument.
Drawing Standard Curves
The certified reference material of stream sediment (GBW07301a and GBW07309), soil (GBW07457), rock (GBW07104), and sample blank were prepared into standard solutions and blank solution, according to the same procedure noted above, with the sample solutions prepared, respectively. The standard curves of ICP-OES and ICP-MS were computed using the standard solutions and the blank solution.
Results and Discussion
Interference and Calibration
Interference and Calibration of ICP-OES
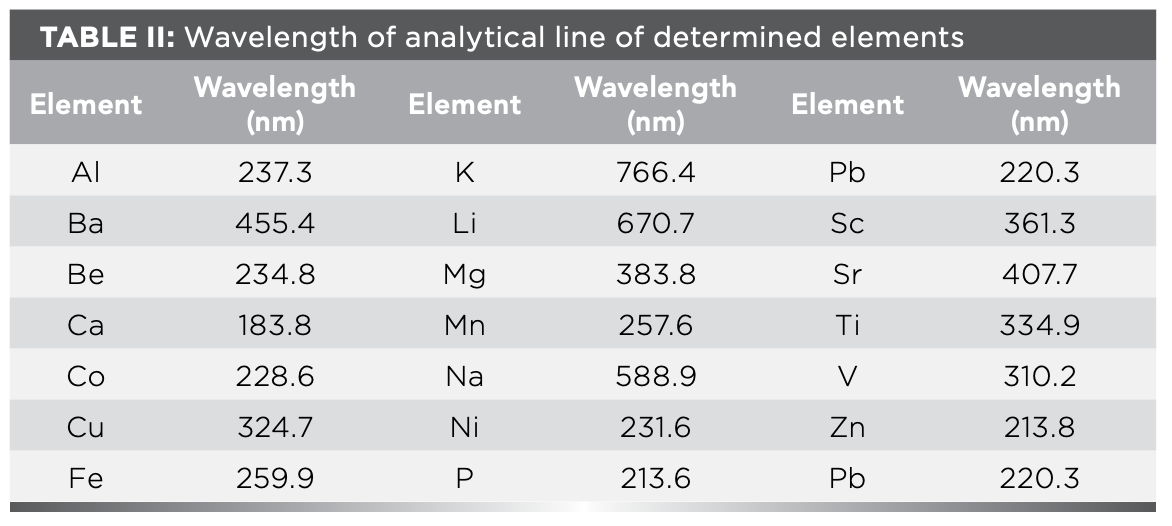
The spectral interference and matrix effect are the main influencing factors in ICP-OES determination. To eliminate matrix effects and spectral line interference, it is necessary to accurately select the analytical spectral lines of each element to be measured. The selection of spectral lines depends on the content of elements; coexistence interference and linear range are mainly considered for major elements, and the detection limit, co-existing interference, and background interference are mainly considered for minor and trace elements (8). Rich characteristic lines of each element are provided in the ICP-OES software. Therefore, the performance of the instrument was used to determine 2–3 selected spectral lines of each element, the background was deducted, and the emission intensity, detection limit, interference of coexisting elements, and linear range of each spectral line were analyzed by an automated function of ICP-OES software. The best spectral line was chosen as the analytical line for analysis. These optimal spectral lines are listed in Table II.
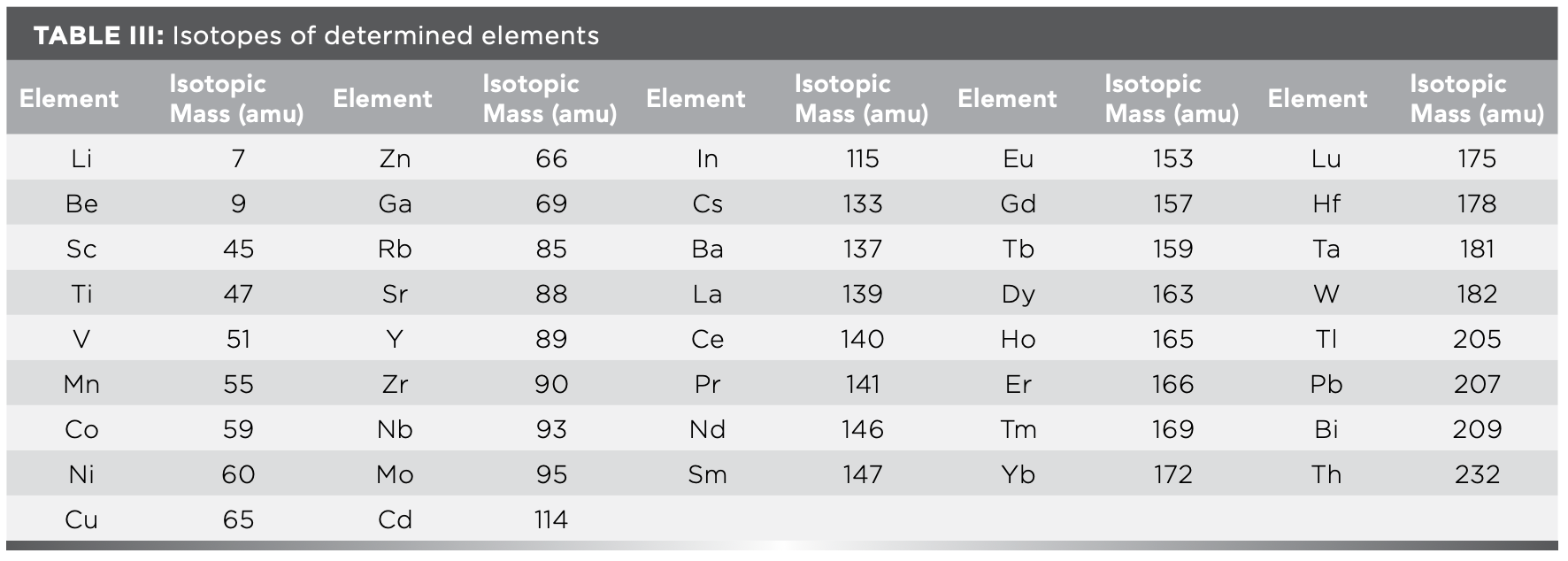
Theoretically, ICP-MS has good selectivity and strong anti-interference ability. However, because of the complexity of geological samples, there are many interferences in ICP-MS analysis, including interference of polyatomic ions, isotopic interference, and matrix effect issues. The interference of polyatomic ions is particularly serious; for example, the interference of light rare earth oxides and hydroxides on heavy rare earth elements, and the interference of oxides of Ba, Ca, and Ti on transition elements. Therefore, during the analysis by ICP-MS, the isotope of element, with less interference, high abundance, and high signal intensity is chosen as the analytical element. The optimal isotopes of determined elements are listed in Table III. At the same time, 185Re (10 μg/L) and 103Rh (10 μg/L) in geological samples were selected as internal standards, and the recovery was controlled within the range of 100% ± 20%, which effectively compensated for the measurement deviation caused by matrix effects.
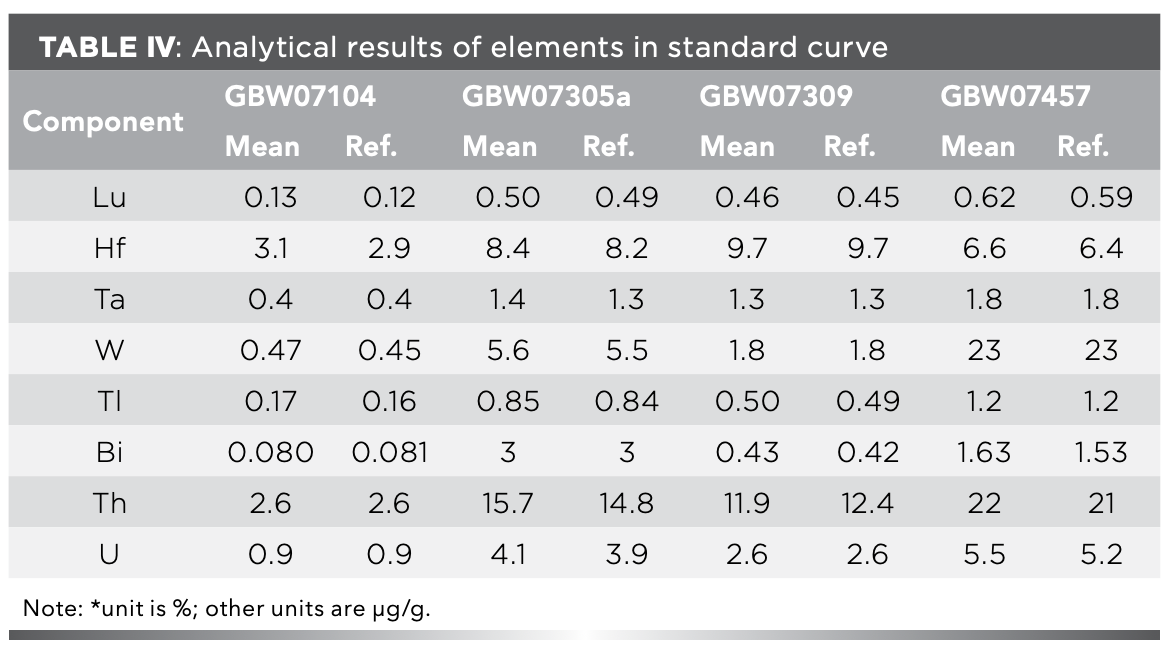
In addition, the standard solution for drawing the standard curve was prepared by using the certified reference materials GBW07301a, GBW07309, GBW07457, and GBW07104 dissolved simultaneously with the samples. This not only ensures that the acidity and matrix element content of the standard and sample are consistent, but it also eliminates the effect of different injection transfer efficiencies caused by different viscosities of solution. Therefore, the matrix effects in the determination of ICP-OES and ICP-MS were further eliminated, and the results were significantly improved. Analytical results of elements using the standard curve are listed in Table Ⅳ.
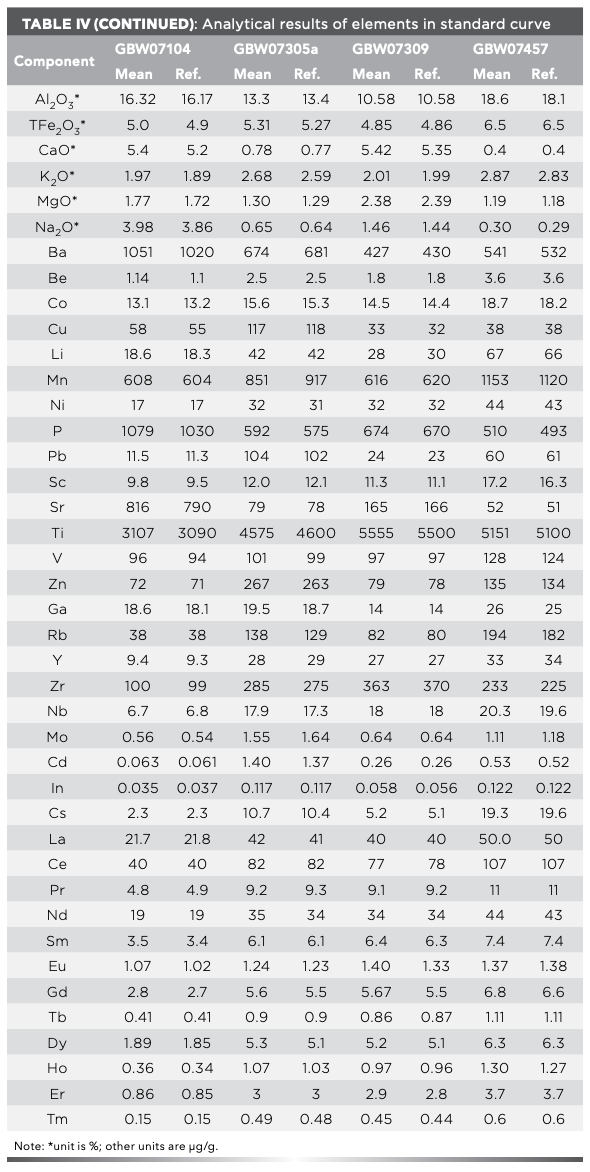
Comparison Between ICP-MS and ICP-OES
The linear dynamic range of ICP-MS and ICP-OES are mg/g to ng/g and x% to 0.0x mg/g, respectively. Therefore, the contents of Ti, V, Mn, Ba, and Sr vary greatly, and the contents of Li, Be, Sc, Co, Ni, Cu, Zn, and Pb vary little in the certified reference material of GBW07301a, GBW07365, GBW07408, GBW07452, GBW07107, GBW07121, and GBW07122 determined simultaneously by ICP-MS and ICP-OES. Figure 1 reveals a good correlation between ICP-MS measured values and ICP-OES measured values.
FIGURE 1: The comparison of the analytical results by ICP-OES and ICP-MS.
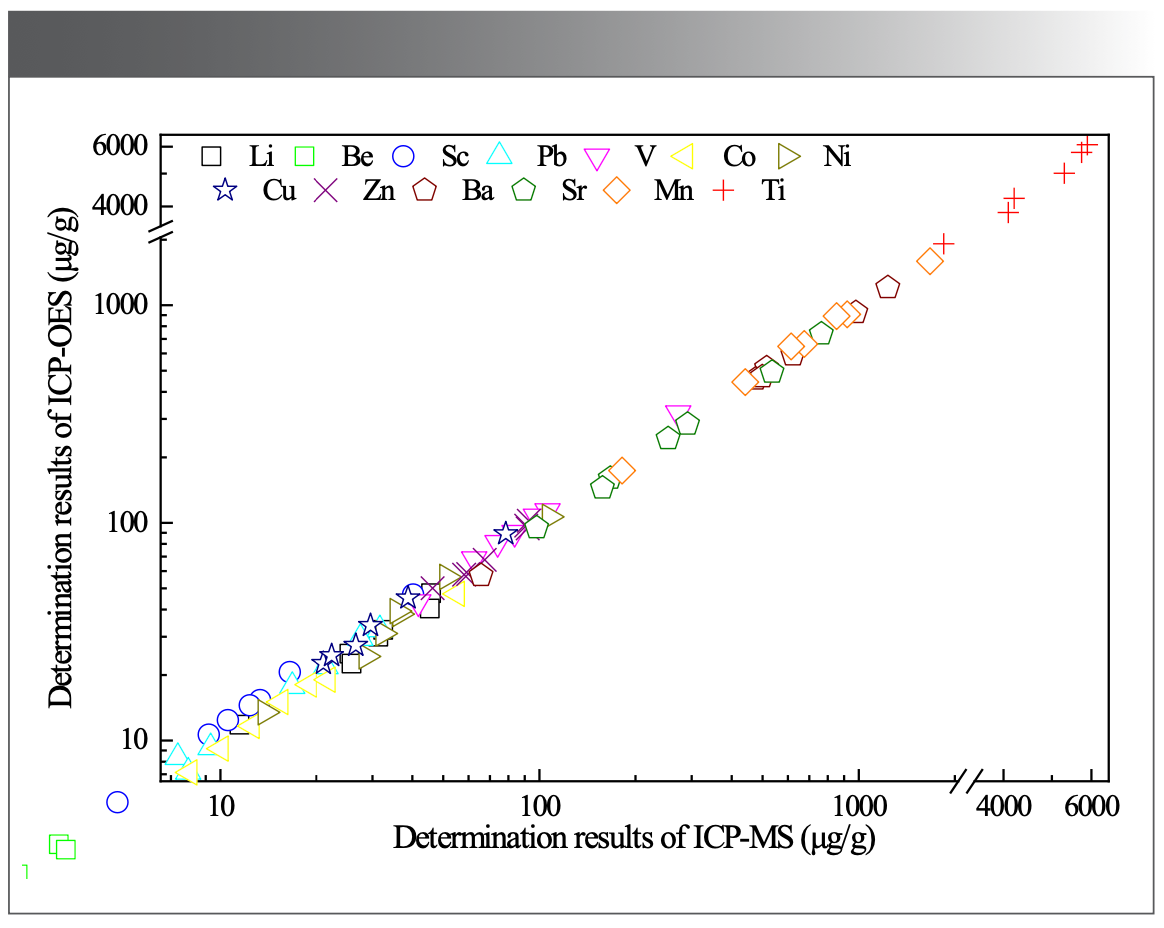
CI-Chondrite Normalized REEs
It is widely known that REEs refer to lanthanide elements (La–Lu) in the periodic table. Two adjacent REEs have different abundances, and their graph of atomic number vs. REEs abundance shows a zigzag pattern. However, chondrite-normalized REEs patterns can eliminate the zigzag pattern and form a smooth curve for REEs, with no significant relative depletions, except for elements Ce and Eu, which would have abnormal behaviors because of the different in valency (Eu[Ⅱ] and Ce[Ⅳ]). Figure 2 shows CI-chondrite normalized REEs abundances for the seven certified reference materials (16), exhibiting smooth chondrite-normalized REE patterns, indicating that our ICP-MS data are reliable.
FIGURE 2: CI normalized distributions of REEs in seven certified reference materials: (R) for recommended value and (M) for determination mean value.
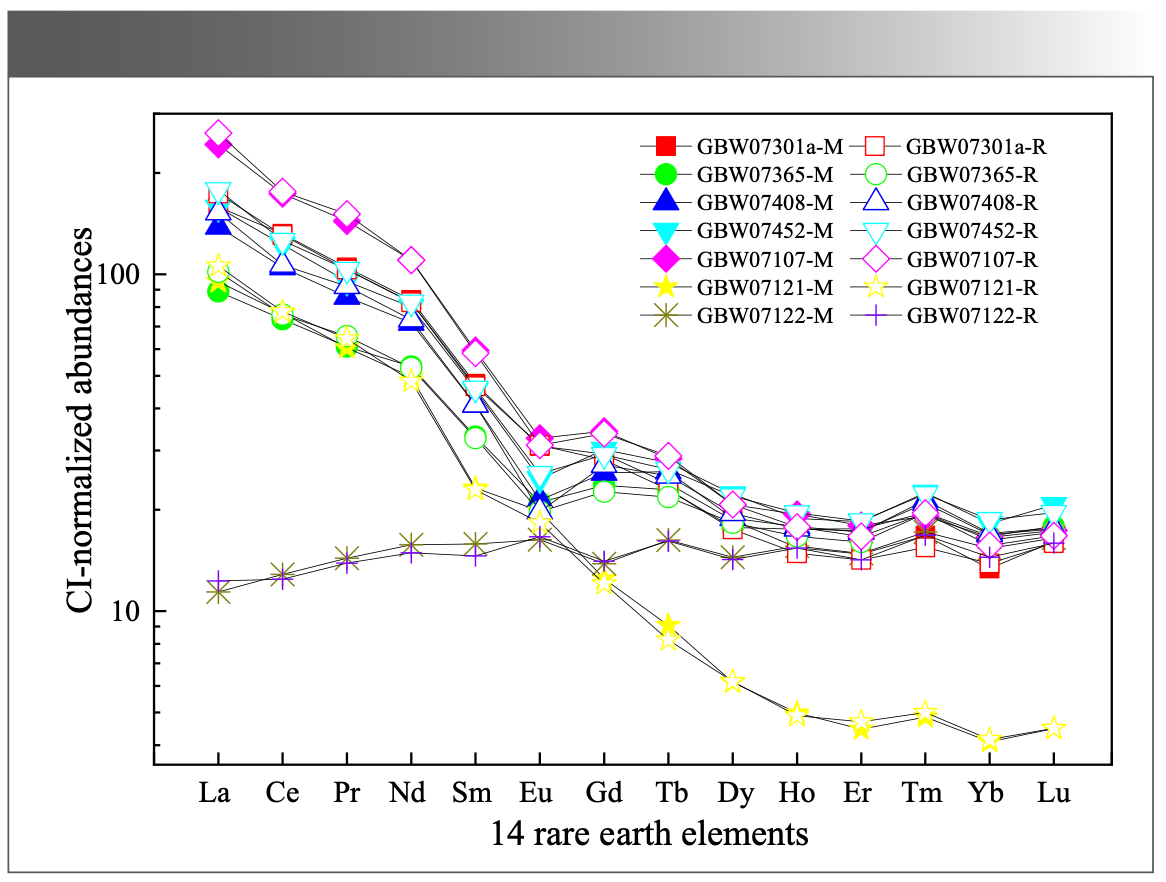
Detection Limit of the Method
According to the procedure of the abovementioned sample preparation method, the limits of detection (LODs) are determined for each element calculated as the concentration of three times the standard deviation from duplicate runs and 12 times that of reagent blank solutions. As illustrated in Figure 3, the LODs values of the ICP-OES are all within the range from 0.2 to 4 μg/g, and that of the ICP-MS are all within the range from 1 to 57 ng/g.
FIGURE 3: Detection limits (LOD) of the method versus element.
Precision and Accuracy of the Method
According to the sample preparation method, the solutions of certified reference materials for stream sediment (GBW07301a and GBW07365), soil (GBW07408 and GBW07452), and rock (GBW07107, GBW07121 and GBW07122) were prepared for 10 parts respectively, and the 51 elements for solutions were detected. The results are presented in Table Ⅴ, and they show that the analytical results are in agreement with the certified values. The precision of the method is shown as the relative standard deviation (RSD) in Figure 4. The RSD in this study is lower than 10% for most elements, except for element Ta in GBW07408 and GBW07122, which are 11.98% and 11.91%, respectively. The accuracy of the method is shown as the relative errors (RE) in Figure 5. It can be seen from Figure 5, the RE in our study are all within the range from -13% to 13% for most elements, except for element Nb in GBW07365, which is 16.03%. The results exhibited good precision and accuracy of the method for all studied elements.
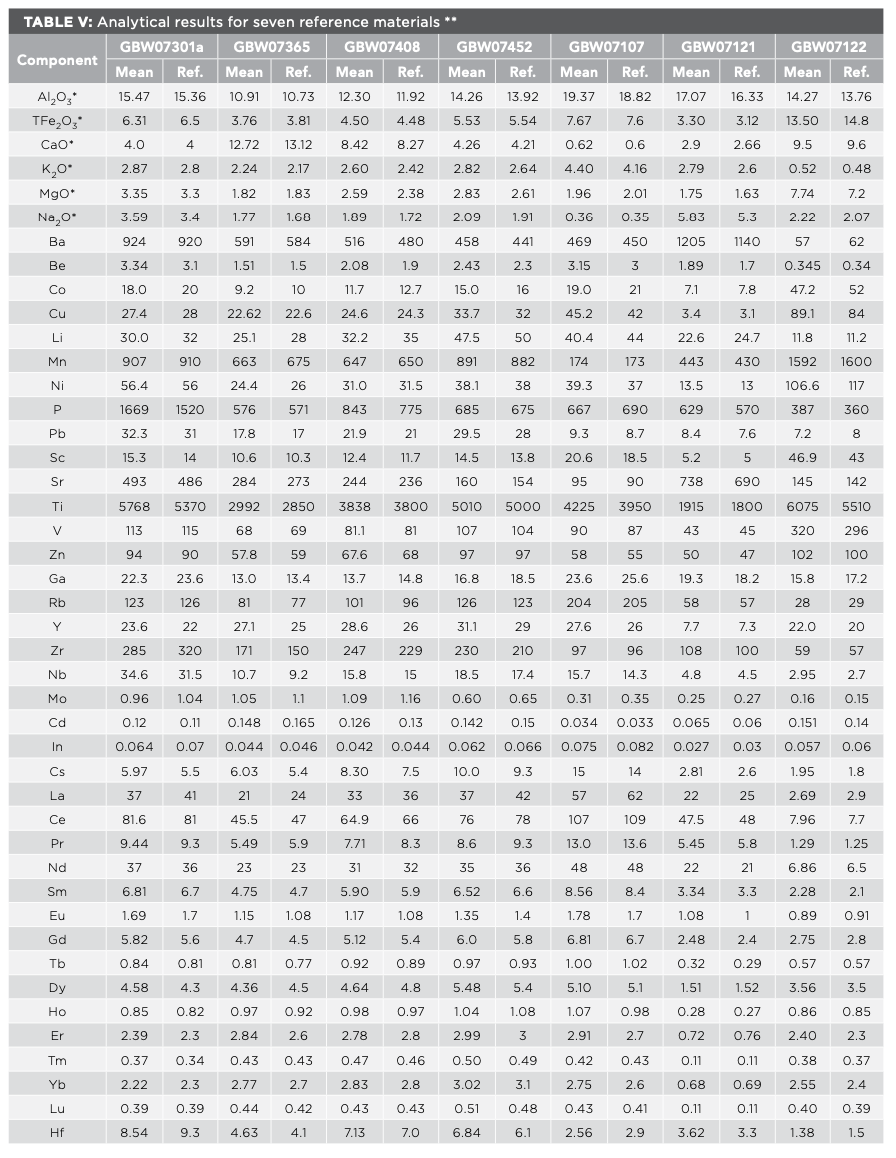
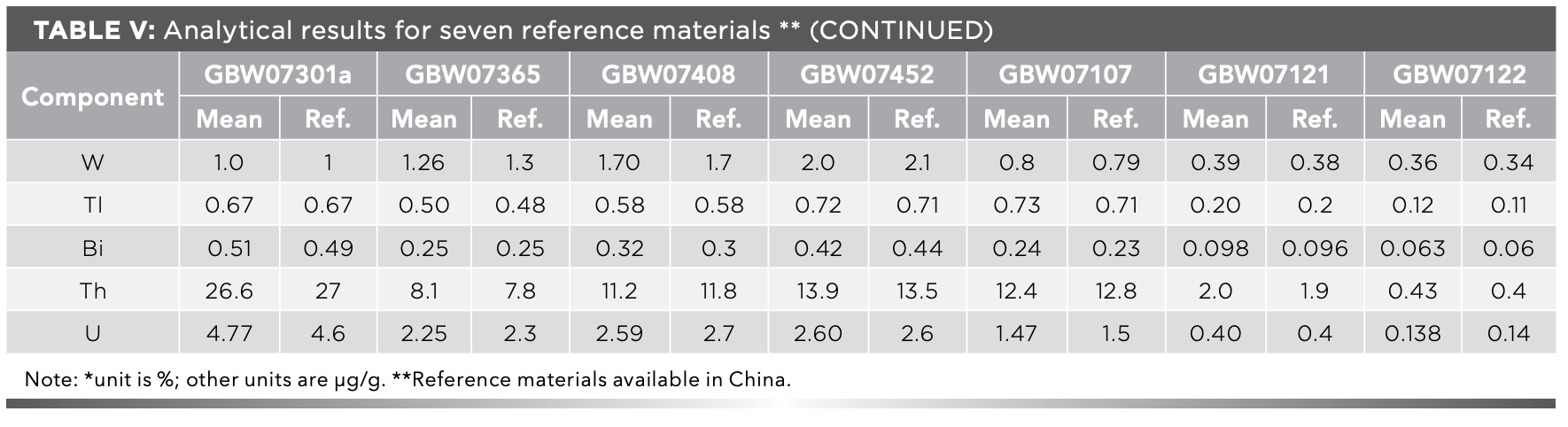
FIGURE 4: Precision of the method per element.
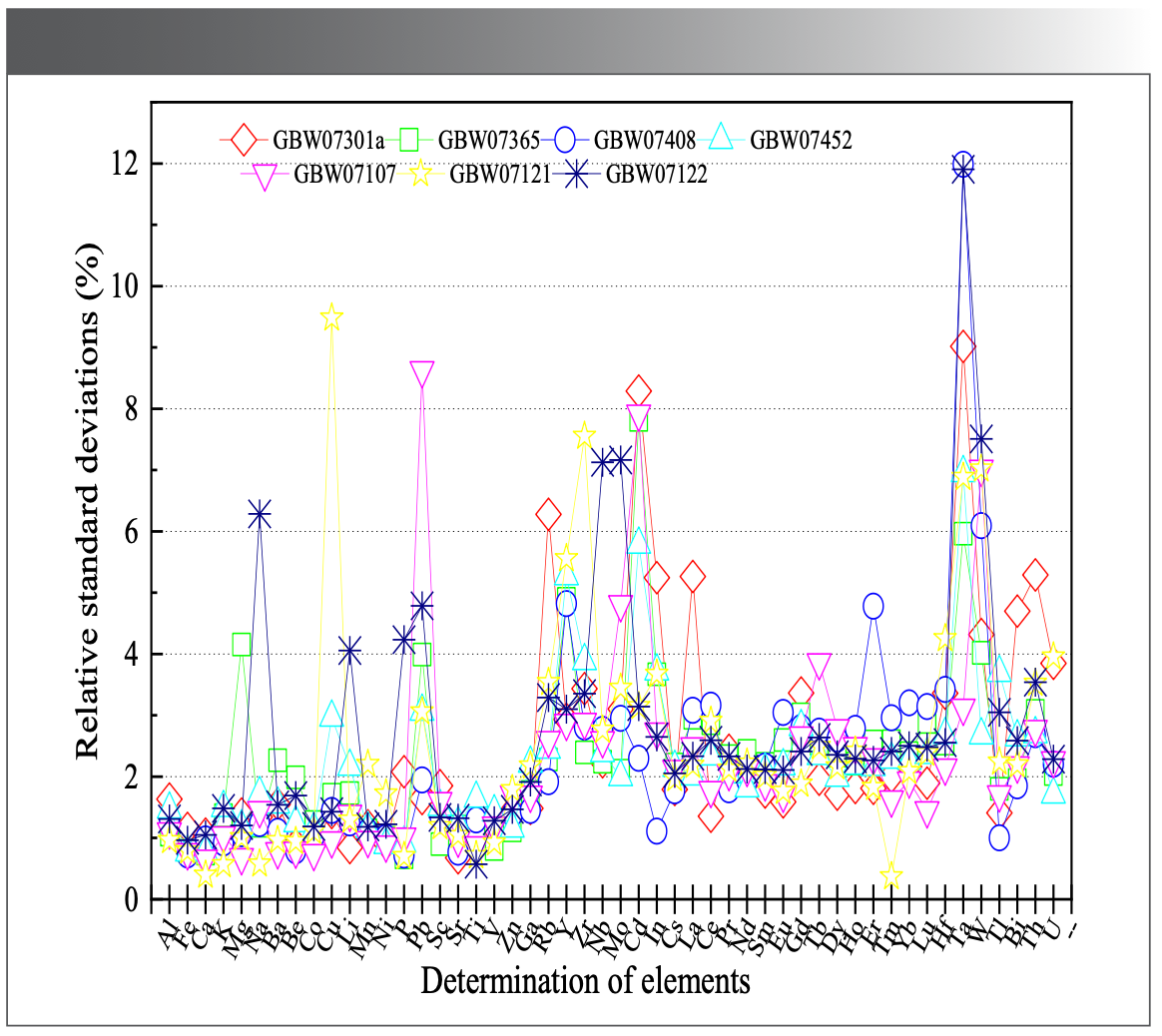
FIGURE 5: Accuracy of the method per element.
Conclusions
Our study developed an improved method using a HCl-HNO3-HF-HClO4 to decompose different type of geological samples, and the method was applied to analyze the content of 50 kinds of major, minor, micro, and trace elements by ICP-OES and ICP-MS. This analytical method was checked by Chinese geological reference materials of stream sediment, soil and rock, and the determined values are in good agreement with the certified values. We conclude that this method has the advantages of low detection limits, good precision, high accuracy, rapidity, and simplicity, which meets the requirements for simultaneous determination of REEs, REs, and other elements of bulk geological samples.
References
(1) Li, G. H.; Fu, G. H.; Tang, X.; Huang, Y.B. Determination of Major, Minor and Trace Elements in Geological Samples with Arsenic by X-Ray Fluorescence Spectrometry. Chin. J. Anal. Lab. 2014, 33, 1220–1224. DOI:10.13595 /j.cnki.issn1000-0720.2014.0286
(2) Gong, C. Determination of Multi-Components in Geological Samples by Energy Dispersive X-ray Fluorescence Spectrometry Coupled with Direct Powder Introduction. Metall. Anal. 2017, 39, 21–28. DOI: 10.13228/j.boyuan.issn1000-7571.009932
(3) Feng, L.S.; Liu, H.Y. Evaluation of Uncertainty of Measurement of Copper in Soil by FAAS. Chin. J. Anal. Lab. 2006, 25, 65–67.
(4) Balaram, V.; Mathur, R.; Satyanarayanan, M.; Sawant, S. S.; Roy, P.; Subramanyam, K.S.V.; Kamala, C.T.; Anjaiah, K.V.; Ramesh, S. L.; Dasaram, B. A Rapid Method for the Determination of Gold in Rocks, Ores and Other Geological Materials by F-AAS and GF-AAS After Separation and Preconcentration by Dibk Extraction for Prospecting Studies. Mapan-J. Metrol. Soc. I. 2012, 27, 87–95. DOI: 10.1007/s12647-012-0012-2
(5) Wang, Y. Z.; Qiu, H. O.; Tang, Z.Y.; Gu, Y. S.; Zhang, S.Q. Determination of Cd in Sediment Samples by Flow Injection On-line Anion Exchange Combined with Vapour Generation-atomic Fluorescence Spectrometry. Spectrosc. Spect. Anal. 2007, 27, 2581–2584.
(6) Zhao, Z. S.; Zhao, X. X.; Jiang, X. X.; Zhao, L.L.; Zhang, L. L. Interference Sources and Elimination Methods for the Determination of Selenium in Soil and Water Sediment by Atomic Fluorescence Spectrometry. Rock Miner Anal. 2019, 38, 333–340. DOI: 10.15898/j.cnki.11-2131/td.201809190106
(7) Shirai, N.; Toktaganov, M.; Takahashi, H.; Yokozuka, Y.; Sekimoto, S.; Ebihara, M. Multielemental Analysis of Korean Geological Reference Samples by INAA, ICP-AES and ICP-MS. J. Radioanal. Nucl. Chem. 2015, 303, 1367–1374. DOI: 10.1007/s10967-014-3653-5
(8) Wen, J. B.; Li, K. Q.; Xiang, Z. B.; Peng, G. P. Simultaneous Determination of Forty Elements in Bauxite by Inductively Coupled Plasma Atomic Emission Spectrometry. Metall. Anal. 2011, 31, 43–49.
(9) Ma, S. F.; Wen, H. L.; Ma, X. R.; Wang, L.; Gong, A. H.; Cao, Y. P.; Qu, W. J. Determination of 22 Elements in Iron, Copper, Zinc, and Lead Sulphide Ores by ICP-AES with Four Acids Digestion. Bulle. Miner Petrol. Geochem. 2011, 30, 65–72.
(10) Zhou, G. X.; Liu, X. X.; Cui, D.S. Determination of 28 Elements Including Rare Earth Elements by ICP-MS in Alkali Melted Rock Sample. J. Chin. Mass Spect. Soc. 2010, 31, 120–124.
(11) Wang, P. P.; Li, X.; Song, W. J. Determination of Rare Earth Elements in Geological Samples by ICP-MS Using Microwave Digestion. J. Instrumental Anal. 2016, 35, 235–240. DOI: 10.3969/j.issn.1004-4957.2016.02.017
(12) Tan, X. J.; Liang, T.; Zhang, Z.; Zhou, Y.; Wang, Y.Q.; Feng, Y. G.; San, J.Z. Quantification Accuracy Study of Lithium in Spodumene Between ICP-MS and ICP-AES. Acta. Geologica. Sinica. 2019, 93, 1543–1549. DOI: 10. 19762/j. cnki.dizhixuebao. 2019164
(13) Liu, Y.; Diwu, C.R.; Zhao, Y.; Liu, X.M.; Yuan, H. L.; Wang, J. Q. Determination of Trace and Rare-Earth Elements in Chinese Soil and Clay Reference Materials by ICP-MS. Chin. J. Geochem. 2014, 33, 95–102. DOI: 10.1007/s11631-014-0663-5
(14) Zhao, W.; Zong, K.Q.; Liu, Y.S.; Hu, Z. C.; Chen, H. H.; Li, M. An Effective Oxide Interference Correction on Sc and REE for Routine Analyses of Geological Samples by Inductively Coupled Plasma-Mass Spectrometry. J. Earth. Sci. 2019, 30, 1302–1310. DOI: 10.1007/s12583-019-0898-5.
(15) Moor, C.; Lymberopoulou, T.; Dietrich, V. J. Determination of Heavy Metals in Soils, Sediments and Geological Materials by ICP-AES and ICP-MS. Mikrochim. Acta. 2001, 136, 123–128.
(16) Anders, E.; Grevesse, N. Abundances of the Elements: Meteoritic and Solar. Geochimica. Cosmochimica. Acta. 1989, 53, 197–214. DOI: 10.1016/0016-7037(89)90286-X
Cang Gong, Haichuan Lu, Kun Zhang, Yang Ding, and Lihua Wang are with the Research Center of Applied Geology of China Geological Survey, in Chengdu, China. Direct correspondence to: dugufengxue@yeah.net ●

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.