Surface-Enhanced Raman Scattering for Biofilm Characterization
Spectroscopy
A summary of the efforts to study microorganism biofilms using surface-enhanced Raman scattering, a powerful technique for in situ analysis of biological molecules and biomolecular organizations
Microorganisms are known as the predominant life form on Earth because of their variability and high metabolic activity. Most of the organisms require protection during their growth after their adhesion onto a surface. The protection layers are known as extracellular polymeric substances (EPS), which include organic based materials such as proteins, carbohydrates, lipids, nucleic acids, and humic-like substances. Microorganisms are found in an embedded form in an EPS matrix, and this whole structure is referred to as a biofilm. The identification and characterization of microorganisms and their growth dynamics based on produced metabolites are very important in fields ranging from the accurate and rapid diagnosis of bacterial infections to industrial processes. Surface-enhanced Raman scattering (SERS) is a powerful technique for identification and characterization of biological molecules and biomolecular organizations. In this review, the effort to use SERS for in situ molecular characterization of biofilms is discussed.
Bacteria can form very large communities because of their high metabolic activity and proliferation rate (1). The genetic material of bacteria is found in the cytoplasm in a suspended form and all metabolic activities take place in the cytoplasm. Bacterial cells are surrounded by a cell wall, which is mostly composed of a peptidoglycan structure. The thickness of the peptidoglycan layer differs depending on the type of bacteria, with Gram-positive bacteria having a thicker layer than Gram-negative bacteria (2).
All bacteria require certain physical and chemical conditions for maximum growth and proliferation. The control of physical and chemical growth conditions, nutrient amount, and competition among microorganisms can be used to prevent unwanted growth. Moreover, control of growth dynamics can provide both the use of metabolic reagents efficiently and the efficient treatment of unwanted activities of bacteria (3). Since infectious bacteria have begun to threaten human health through epidemic diseases and bioterrorism, the growth dynamics of bacteria have been investigated through broader means with conventional and novel technologies.
Bacterial growth is commonly associated with the formation of a protective layer, which is composed of extracellular polymeric substances (EPS) and water channels that serve as a permeable barrier. The biofilm structure, which includes both the bacteria and EPS, provides protected growth within strict environmental conditions, even in the presence of antimicrobial agents. The biofilm structure also allows for genetic diversity through processes such as horizontal gene transfer. The type of microbial community, nutrient availability, and surface properties where bacteria adhere, result in diversity in biofilm structure (4–6). Because of the complex nature and diversity in biofilm structures, in situ characterization of biofilm structures at the molecular level is a challenge and an important issue in various fields including medicine and industrial processes.
Current Techniques for the Characterization and Identification of Biofilms
To characterize and identify microbial growth and growth-associated structures such as biofilms at the molecular level, several different techniques have been developed over the years. Conventional approaches such as phenotypic and serological tests, fatty acid profiles, protein profiling, nucleic acid probing, methods based on examination, and staining are routinely used for biofilm characterization. Although they provide valuable information, these methods are time consuming and require highly trained personnel most of the time (7,8). As alternatives, spectroscopic approaches such as mass spectrometry (MS) and vibrational spectroscopic techniques have been investigated in recent years. It was shown that spectroscopic techniques were very suitable for characterization and identification of components of complex biological structures such as microbial mass and biofilms (9). MS and infrared (IR) spectroscopy techniques can offer additional benefits such as molecular-level information and easy sample preparation. However, both techniques have certain drawbacks. For example, MS is an expensive technique and requires trained personnel. IR is overly sensitive for water and has limited spatial resolution (10–13).
Most of the previous studies to determine the complex structure of biofilms at the molecular level were based on visualization techniques with staining steps. Confocal laser scanning microscopy (CLSM), optical coherence tomography (OCT), and transmission electron microscopy (TEM) are examples for these techniques (14–18). However, the highly complex nature of biofilms and difficult protocol development for CLSM and OCT limit their use. Although TEM is highly informative to identify biofilm structure, the integrity of biofilms may change during sample preparation and transfer of bacteria from one place to another, which may decrease the reliability of results (19–21). Vibrational spectroscopic techniques such as Fourier transform infrared (FT-IR) and Raman spectroscopy can also be used for the characterization of complex molecular structure of biofilms (22–24). Although both techniques provide molecular level information about biofilm structures, FT-IR suffers from limited spatial resolution and water in samples as mentioned earlier.
Raman spectroscopy has been extensively investigated to gather molecular information from complex biological systems such as eukaryotic cells (25,26), microorganisms (27), and tissues (28). The advantages, such as easy sample preparation, almost immunity to water, and narrow spectral bandwidths, make the technique more suitable for biological applications. However, similar to other techniques, Raman spectroscopy also has disadvantages. The most significant disadvantage of the technique is the weak scattering pertaining to the nature of Raman scattering phenomena. The inherently weak Raman scattering requires a powerful light source, a sensitive detector, and increased spectral collection time. With the discovery of a phenomenon called surface-enhanced Raman scattering (SERS), in which the Raman scattering can be enhanced up to 1014 by bringing the molecule or molecular structures into close proximity of a nanostructured noble metal surface (29–32). The enhancement of Raman scattering from pyridine on a silver electrode was first observed by Fleischmann and colleagues (33). Later, the mechanism behind this enhancement was independently explained with an electromagnetic effect by Jeanmaire and Van Duyne (57) and with a charge-transfer effect between the noble metal surface and molecule by Albrecht and Creighton (58). It is now generally accepted that the enhancement mechanism has two components: electromagnetic and charge transfer (30,34–36). Electromagnetic theory is based on the formation of surface plasmons that is localized around the noble metal nanostructures. When a molecule interacts with the plasmonic area on the nanoparticle surface, the induced dipole moment and inelastic collision increase (37). In a charge-transfer mechanism, movement of electrons between the metal and molecule is responsible for the enhanced scattering. The contribution of the electromagnetic component (104 –107) to the overall enhancement is considered more than the enhancement originating from the charge transfer (10–102) (36).
The type of SERS substrates can vary from colloidal particles to rationally designed nanostructures depending on the nature of the application. Although the nanostructured metals such as Au, Ag, Cu, and Al support surface plasmons in the visible and near-infrared regions (300–1200 nm), Au and Ag are the most commonly used metals to construct SERS substrates because of their inertness, easy preparation, and high enhancement factors in the visible region of the spectrum. Among the SERS substrates, silver nanoparticles are widely used because of their easy preparation, low cost, and high enhancement properties. Although the silver nanoparticles can be prepared with several methods such as reduction with organic and inorganic agents (38,39), ultrasound, UV light, and gamma rays (40), the citrate reduction method is one of the widely used methods in SERS applications, again because of their simple preparation.
Since the early days of its discovery, an increasing trend in the application of SERS for the solution of biological problems has been observed. The technique was used for the identification, characterization, and detection of biological structures and species including DNA, proteins, lipid, cells, tissues, viruses, bacteria, and yeast by using various types of SERS substrates and nanostructured surfaces (41–47).
SERS and Biofilm Characterization
The previous research on the topic demonstrated the power of the technique for the analysis of complex biological samples such as bacteria, yeast, cell-wall components, and biofilm structures (41–48). Holt and Cotton (49) were the first to demonstrate the feasibility of SERS for microbial research. Different SERS substrates and sample preparation methods were attempted for the identification and characterization of bacteria in the following years (50–52).
The use of SERS was also investigated for biofilm characterization. Ivleva and colleagues (21) first demonstrated the applicability of SERS by obtaining reproducible spectra from multispecies biofilms. In their study, hydroxylamine hydrochloride–reduced colloidal silver nanoparticles were used as SERS substrates and the analysis of biofilm components was demonstrated by comparing SERS and Raman spectroscopic techniques, showing the suitability of SERS by monitoring the intensities of the selected bands on the spectra. In another study by the same group, the sensitivity of SERS for the detection of low concentrations of biomolecular components of a biofilm was demonstrated. In addition, the results obtained from CLSM were compared to the results obtained from the SERS study. It was found that the performance of SERS was superior to that of CLSM (19).
In a study by Ramya and colleagues (53), the growth of biofilms on titanium surfaces was investigated to determine EPS constituents in the biofilm formed by algae and Pseudomonas aeruginasa using Raman spectroscopy. In addition, in the same study SERS was used with colloidal Ag and CuNPs as SERS substrates. When the chemical composition of biofilm structures from algae and bacteria were compared, the different amounts of constituents in both types of biofilms using Raman spectroscopy and SERS were observed on Ti surfaces. In most biofilm characterization studies, the colloidal silver nanoparticles are prepared by reduction of Ag+ ions with a convenient reducing reagent such as sodium citrate or borohydrate, and then are used as SERS substrates. In a more recent report, we demonstrated an in-situ biofilm monitoring approach by using a core–shell type SERS substrate (54). In situ characterization prevents the possibility of undesired interferences during sample handling, such as washing, filtering, and staining microorganisms. In our study, the citrate-reduced colloidal silver nanoparticles coated with a thin layer of chitosan were used. All SERS measurements were carried out with an InVia Reflex model Raman microscopy system (Renishaw). The system was automatically calibrated against a silicon wafer band at 520 cm-1. A diode laser at 830 nm with a laser power of 3 mW and 50× objective was used in all measurements. Our study demonstrates that this new substrate, chitosan-coated silver nanoparticles, is highly useful for the characterization of negatively charged species in a biofilm composition because of the free amino groups in the chitosan structure. The nature of the interaction between chitosan and silver nanoparticles is thought to be electrostatic, and positively charged chitosan adheres to the negatively charged silver nanoparticles because of the presence of citrate ions on their surfaces. Chitosan on the surface of the silver nanoparticles acts not only as a selective barrier, but also prevents the release of Ag+ ions into the complex biofilm structure, which might have an influence on the bacterial growth. Escherichia coli (E. coli) and Staphylococcus cohnii (S. cohnii) were used as model microorganisms in the study. The bacteria were incubated for 3–48 h and their metabolic activities were monitored by means of SERS spectra during their growth. Figure 1 shows the SERS spectra of biofilm formations for E. coli (Figure 1a) and S. cohnii (Figure 1b). The bands marked with an asterisk show intensity variations during the bacterial growth. Note that the spectra are normalized for intensity comparison. Figure 1c shows the tentative band assignment for the bands in which the most dramatic changes were observed.
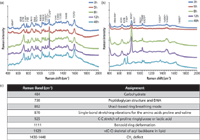
Figure 1: SERS spectra of biofilm formed by (a) E. coli and (b) S. cohnii during their growth from 3 to 48 h and (c) tentative assignments of distinct bands. Adapted from reference 54.
The chemical changes in the bacterial cell wall with exposure to antibiotics were demonstrated by Liu and colleagues (55). In their study, Ag/anodic aluminum oxide (AAO) substrate was used to develop a diagnostic platform for the rapid and accurate diagnosis of bacteria. As an ongoing effort to utilize SERS in microbiological applications, we recently became interested in the observation of bacterial death based on molecular changes with antibiotics exposure. We tested the approach using E. coli as a model microorganism and ampicillin. Chitosan-coated silver nanoparticles were used again as the SERS substrate. We found that SERS was a powerful technique to monitor the molecular level changes at the bacterial cell wall and in biofilm compositions.
The spectral changes observed on the SERS spectra during the bacterial death were evaluated in the presence of ampicillin antibiotic (0.2 mg/mL). Firstly, E.coli were inoculated onto nutrient agar and incubated for 8 h until they reach the log phase. A 5-µL volume of ampicillin solution was dropped onto the 8-h incubated E.coli culture plate. A 10-µL volume of chitosan-coated silver nanoparticle suspension was placed onto the area, which includes both ampicillin and bacteria, and they were incubated for 10 min to provide maximum interaction between the chitosan-coated silver nanoparticles and the media components. The SERS spectra obtained during the death of bacteria are shown in Figure 2. The SERS acquisition was performed at the end of the second hour because we could not observe any change in the SERS spectra. Therefore, the SERS spectra acquisitions were continued at the end of the fourth and sixth hours. SERS revealed that a significant change in the biochemical composition of the environment of bacteria took place as the bacteria were exposed to antibiotics for longer times. The biochemical changes in the environment are clearly reflected by the SERS spectra. Although several new peaks appear on the SERS spectra at the end of the sixth hour, the emergence of a very intense peak at 678 cm-1 confirms the significant changes in the biochemical composition on the surface of the culture plate. This band can be attributed to DNA or RNA released from bacterial cells as they continue to disintegrate (56). As mentioned before, the chitosan-coated silver nanoparticles act as an attraction center for negatively charged molecules. DNA and RNA have a highly dense negative charge in their structure because of the phosphate groups. Thus, when bacteria lose their integrity as a result of antibiotic treatment, genetic material leaks out and comes into contact with the silver nanoparticle surfaces.
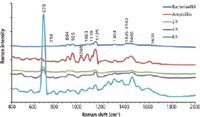
Figure 2: SERS spectra obtained during the death of E. coli with ampicillin exposure. NA: Nutrient agar.
Conclusions
This review provides a summary of the efforts to use SERS for biofilm characterization. Although only a limited number of reports are available in the literature, from previous studies as well as our own, it is plausible to say that SERS is an emerging technique to study complex biological structures such as biofilms. We also demonstrated that the use of chitosan-coated silver nanoparticle substrates could be an efficient way to characterize molecular constituents in biofilm structures. In future studies, the use of rationally designed SERS substrates in biofilm characterization studies can be considered for more reliable and detailed information.
Acknowledgments
The authors gratefully acknowledge the financial support of Yeditepe University and The Scientific and Technological Council of Turkey (TUBITAK) during the course of this study.
References
(1) R.M. Atlas, Microorganisms in Our World (Mosby Year Book, in C. Wm, Missouri, 1995).
(2) W. Vollmer, D. Blanot, and M.A. de Pedro, FEMS Microbiol Rev.32, 149–167 (2008).
(3) L. McKane and J. Kandel, Bacterial Growth and Laboratory Cultivation (McGraw-Hill, New York, 1996).
(4) R.M. Donlan, Healthcare Epidemiology33, 1387–1392 (2001).
(5) P. Stoodley, J.D. Boyle, I. Dodds, and H.M. Lappin-Scott, BioLine (Cardiff, UK ,1997) pp. 1–9.
(6) J.W. Costerton, Z. Lewandowski, D.E. Caldwell, D.R. Korber, and H.M. Lappin-Scott, Annu. Rev. Microbiol.49, 711–745 (1995).
(7) D. Cam, K. Keseroglu, M. Kahraman, F. Sahin, and M. Culha, J. Raman Spectrosc. doi: 10.1002/jrs.2475 (2009).
(8) S.L.W. On, Clin. Microbiol. Rev.9, 405–422 (1996).
(9) S. Efrima and L. Zeiri, J. Raman Spectrosc.40, 277–288 (2009).
(10) M.A. Claydon, S.N. Davey, V. Edwards-Jones, and D.B. Gordon, Nat. Biotechnol. 14, 1584–1586 (1996).
(11) A.M. Haag, S.N. Taylor, K.H. Johnston, and R.B. Cole, J. Mass. Spectrom.33, 750–756 (1998).
(12) M. Kahraman, M. Yazici, F. Sahin, and M. Culha, Langmuir. 24, 894–901 (2008).
(13) D. Naumann, Proceedings of SPIE - The International Society for Optical Engineering3257, 245–257 (1998).
(14) T. Zhang and H.H.P. Fang, Biotechnol Lett.23, 405–409 (2001).
(15) J.R. Lawrence, T.R. Neu, and G.D.W. Swerhone, J. Microbiol. Methods32, 253–261 (1998).
(16) C. Haisch and R. Niessner, Water Res.41, 2467–2472 (2007).
(17) T.A. Smirnova, L.V. Didenko, I.G. Tiganova, S.G. Andreevskaya, N.V. Alekseeva, T.V. Stepanova, and Y.M. Romanova, Appl. Biochem. Microbiol.46, 706–711 (2010).
(18) R.C. Hunter and T.J. Beveridge, J. Bacteriol.187, 7619–7630 (2005).
(19) M. Wagner, N.P. Ivleva, C. Haisch, R. Niessner, and H. Horn, Water Res.43, 63–76, (2009).
(20) N.P. Ivleva, M. Wagner, A. Szkola, H. Horn, R. Niessner, and C. Haisch, J. Phys. Chem. B. 114, 10184–10194 (2010).
(21) N.P. Ivleva, M. Wagner, H. Horn, R. Niessner, and C. Haisch, Anal. Chem. 80, 8538–8544 (2008).
(22) N.A. Ngo Thi and D. Naumann, Anal. Bioanal. Chem.387, 1769–1777 (2007).
(23) H.N.N. Venkata, N. Nomura, and S. Shigeto, J. Raman Spectrosc.42, 1913–1915 (2011).
(24) T. Schmid, A. Sebesta, J. Stadler, L. Opilik, R.M. Balabin, and R. Zenobi, Proc. of SPIE 7586 (2010).
(25) P. Rosch, M. Harz, M. Schmitt, and J. Popp, J. Raman Spectros.36, 377–379 (2005).
(26) P. Rösch, M. Harz, K.D. Peschke, O. Ronneberger, H. Burkhardt, and J. Popp, Biopolymers82, 312–316 (2006).
(27) R.M. Jarvis and R. Goodacre, J. Anal. Chem. 76, 40–47 (2004).
(28) A. Nijssen, T.C. Bakker Schut, F. Heule, P.J. Caspers, D.P. Hayes, M.H.A. Neumann, and G.J. Puppels, J. Invest. Dermatol. 119, 64–69 (2002).
(29) M. Moskovits and D.P. Dillella, in Spectroelectrochemistry: Theory and Practice, R.K. Chang and T.E. Furtak, Eds. (Plenum Press, New York, 1982), pp. 243–273.
(30) M. Moskovits, Rev. Mod. Phys. 57, 783–826 (1985).
(31) M. Kahraman, M. Yazici, F. Sahin, O.F. Bayrak, and M. Culha, Appl. Spectrosc. 61, 479–485 (2007).
(32) S. Abald Cela, P. Aldeanueva Potel, C. Mateo, L. Rodri'guez Lorenzo, R.A. Alvarez Puebla, and L.M. Marzan, J. R. Soc. Interface.7, 435–450 (2010).
(33) M. Fleischmann, P.J. Hendra, and A.J. McQuillan, Chem. Phys. Lett. 26,(2), 163–166 (1974).
(34) A. Campion and P. Kambhampati, Chem. Soc. Rev. 27, 241–250 (1988).
(35) M. Moskovits, J. Chem. Phys.69, 1459–1461 (1978).
(36) B.N.J. Persson, Chem. Phys. Lett.82, 561–565 (1981).
(37) C. Haynes, A.D. Mcfarland, and R.P. Van Duyne, Anal. Chem.77, 339A–346A (2005).
(38) S.H. Chen and D.L. Carroll, Nano Lett.2, 1003–1007 (2002).
(39) Y.G. Sun and Y.N. Xia, Science298, 2176–2179 (2002).
(40) R. Prucek, L. Kvitek, and J. Hrbac, Acta Univ. Palacki. Olom. Chemica.43, 7–27 (2004).
(41) M. Culha, M. Kahraman, D. Cam, I. Sayin, and K. Keseroglu, Surf. Interface Anal. doi.10.1002/sia.3256 (2010).
(42) O. Aydin, M. Altas, M. Kahraman, O.F. Bayrak, and M. Culha, Appl. Spectrosc.63, 1095–1100 (2009).
(43) K. Kneipp, A.S. Haka, H. Kneipp, K. Badizadegan, N. Yoshizawa, C. Boone, K.E. Shafer-Peltier, J.T. Motz, R.R. Dasari, and M.S. Feld, Appl. Spectrosc.56, 150–154 (2002).
(44) W. Ren, J.Y. Liu, S. Guo, and E. Wang, Science China Chemistry54, 1334–1341 (2011).
(45) S. Shanmukh, L. Jones, J. Driskell, Y. Zhao, R. Dluhy, and R.A. Tripp, Nano Lett.6, 2630–2636 (2006).
(46) F. Ni, R. Sheng, and T.M. Cotton, Anal. Chem. 62, 1958–1963 (1990).
(47) S. Keskin and M. Culha, Analyst137, 2651–2657 (2012).
(48) L. Zeiri, B.V. Bronk, Y. Shabtai, J. Eichler, and S. Efrima, Appl. Spectrosc.58, 33–40 (2004).
(49) R.E. Holt and T.M. Cotton, J. Am. Chem. Soc.111, 2815–2821 (1989).
(50) A. Sengupta, M.L. Laucks, and E.J. Davis, Appl. Spectrosc. 59, 1016–1023 (2005).
(51) R.M. Jarvis, A. Brooker, and R. Goodacre, Anal. Chem.76, 5198–5202 (2004).
(52) M. Kahraman, M.M. Yazici, F. Sahin, and M. Culha, Langmuir24, 894–901 (2008).
(53) S. Ramya, R.P. George, R.V. Subba Rao, and R.K. Dayal, Appl. Surf. Sci. 256, 5108–5115 (2010).
(54) E. Efeoglu and M. Culha, Appl. Spectrosc.67, 498–505 (2013).
(55) T.T. Liu, Y.H. Lin, C.S. Hung, T.J. Liu, Y. Chen, Y.C. Huang, T.H. Tsai, H.H. Wang, D.W. Wang, J.K. Wang, Y.L. Wang, and C. Hu. Lin, Plos one4, e5470 (2009).
(56) Z. Movasaghi, S. Rehman, and I.U. Rehman, Appl. Spectrosc. Rev.42, 493–541 (2007).
(57) D.L. Jeanmaire and R.P. Van Duyne, Journal of Electroanal. Chem. 84, 1–20 (1977).
(58) M.G. Albrecht and J. Creighton, J. Am. Chem. Soc. 99(15), 5215–5217 (1977).
Esen Efeoglu and Mustafa Culha are with the Department of Genetics and Bioengineering, Faculty of Engineering, at Yeditepe University, in Atasehir, Istanbul, Turkey. Direct correspondence to: mculha2@gmail.com

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.