Tandem Mass Spectrometry Improves ICP-MS Detection Limits and Accuracy for Trace Level Analysis of Titanium
Special Issues
Titanium (Ti) is used in lightweight alloys, catalysts, cutting and grinding tools, and medical implants. Most Ti is refined into the white pigment titanium dioxide (TiO2), used in paint, paper, plastics and food, and in consumer products such as toothpaste and sunscreens. TiO2 is considered inert and safe, but TiO2 nanoparticles are much more reactive and bioavailable, so they may have adverse effects on human health and the environment. There is a need for analytical techniques and methods for accurate, low level analysis of Ti in environmental samples, biological tissues, and other samples. This application is difficult for conventional quadrupole ICP-MS because of the presence of matrix-based spectral interferences on the isotopes of Ti. In this paper, we show how the interferences can be removed using tandem ICP-MS (ICP-MS-MS). This allows sub-ng/L (ppt) detection limits to be achieved, and ensures that accuracy is maintained in the presence of complex sample matrices
Titanium (Ti) is used in lightweight alloys, catalysts, cutting and grinding tools, and medical implants. Most Ti is refined into the white pigment titanium dioxide (TiO2), used in paint, paper, plastics, food, and consumer products such as toothpaste and sunscreens. TiO2is considered inert and safe, but TiO2nanoparticles are much more reactive and bioavailable, so they may have adverse effects on human health and the environment. There is a need for analytical techniques and methods for accurate, low level analysis of Ti in environmental samples, biological tissues, and other samples. This application is difficult for conventional quadrupole inductively coupled plasma-mass spectrometry (ICP-MS) because of the presence of matrix-based spectral interferences on the isotopes of Ti. In this article, we show how the interferences can be removed using tandem ICP-MS (ICP-MS-MS). This approach allows sub-nanogram-per-liter (part-per-trillion) detection limits to be achieved and ensures that accuracy is maintained in the presence of complex sample matrices.
Titanium (Ti) is an important element for high-performance alloys used in automotive, consumer, and medical products, and especially in aerospace applications, where light weight, high strength, and tolerance of extremely high temperatures are important factors. However, most titanium is refined into titanium dioxide powder (TiO2), which is used as a whitener or anticaking agent in industrial and consumer products such as paints, paper, plastics, food, cosmetics, and sunscreen. Fine TiO2 particles (>0.1 mm in diameter) are known as pigment grade, and smaller (<0.1 mm) ultrafine TiO2 particles are classified as nanoparticles. Although TiO2 powder is considered a poorly soluble, low-toxicity (PSLT) material, there is some evidence of it causing lung damage including tumor growth from respiratory exposure, with damaging effects being greater for smaller particles (1). Ti is also used in medical devices and implants such as joint replacements, so methods are needed to monitor the possible degradation of these implants.
Quadrupole inductively coupled plasma–mass spectrometry (ICP-QMS) is a standard technique for analyzing trace metals in many sample types including environmental, food, and biological materials. ICP-MS can measure Ti at low levels, although three of its five isotopes (46Ti, 48Ti, and 50Ti) are overlapped by minor isotopes of other elements (46Ca, 48Ca, 50V, and 50Cr). The minor isotopes 47Ti (7.44% abundance) and 49Ti (5.41% abundance) are therefore typically used for Ti quantitation by ICP-MS, so sensitivity and detection limits are poorer than they would be if a major isotope was available. In sample matrices that contain high levels of Ca, P, C, Cl, or S, common matrix elements in many natural materials, all of the Ti isotopes can also be affected by polyatomic interferences including CaH+, PO+, SOH+, and CCl+ (2).
ICP-QMS with collision-reaction cell (CRC) technology operating in helium (He) mode is able to reduce most common polyatomic overlaps to background or negligible levels (3). But small residual interferences may remain, affecting the measurement of certain analytes at trace levels in some matrices. The presence of residual interferences has led to interest in using reactive cell gases to improve interference removal and provide lower detection limits for specific elements.
Reaction gas methods can use either on-mass or mass-shift measurement. On-mass measurement is used when the interfering ions are more reactive than the analyte ion; the interferences are neutralized or react to form new product ions at a different mass, leaving the analyte ion free to be measured at its original mass. Mass shift mode is used when the analyte ion is more reactive than the interferences; the analyte is measured as a new, cell-formed product ion, free from the original overlap. Either of these reaction cell modes can be used with conventional ICP-QMS, but the single mass analyzer configuration does not provide any control of the ions that enter the cell. As a result, all ions are able to react with the cell gas, leading to the formation of many new product ions, some of which will overlap the analyte ions of interest. Even the presence of low mass ions such as Ar+ and O+, which are ubiquitous in the ICP-MS ion beam in solution analysis, will lead to reaction product ions that may overlap the higher masses where analyte product ions are measured, especially when a highly reactive cell gas such as ammonia (NH3) is used (4). In practice, this means that the analyte product ions measured in reaction mode with ICP-QMS may in fact consist of several different product ions derived from different analyte isotopes, and other analyte, matrix and solvent or plasma gas elements. The errors caused may not be apparent during method development, since the product ions will vary depending on the matrix. Also, reaction chemistry with ICP-QMS is usually specific to one isotope of the analyte, so there is often no independent confirmation of the result based on a secondary or qualifier ion, as commonly used in organic mass spectrometry, and ICP-MS with He cell gas.
An ICP-QMS system with a quadrupole ion guide in the cell can reject masses above and below a user-set mass. This approach, known as a bandpass filter, can be used to reject low (and high) mass ions that might react in the cell to form new interfering ions, but the mass window limits cannot be set very close to the mass of the analyte precursor ion, or severe loss of analyte sensitivity occurs (5). Thus, bandpass operation cannot reject existing ions at the intended analyte product ion mass, nor can it differentiate between reaction product ions that occur at the same mass, but are formed from different isotopes or elements. An example might be the overlap of the NH3 product ions [63Cu(NH3)3]+ and [64ZnNH2(NH3)2]+, which occur at the same mass (m/z 114) as the product ion [48TiNH(NH3)3]+. This effect is apparent in the poor isotopic template fit observed when the reaction product ions from several isotopes of the same element are measured in ICP-QMS (6).
MS-MS mode is an alternative mode of operation available on a triple-quadrupole ICP-MS. With MS-MS, the ions that enter the cell are controlled by an additional quadrupole mass spectrometer (Q1), situated before the CRC. Q1 can be operated as a mass filter, allowing only the target analyte ions and any on-mass interferences to enter the cell. This gives far better control of reaction processes, and allows the use of highly reactive cell gases without the matrix-dependent variability that occurs with ICP-QMS. A further benefit of MS-MS mode is that, since each analyte ion enters the cell in isolation (without any other isotopes of the same element), and the mass shift transition is specific, as defined by the mass difference between Q1 and Q2 (for example, + 16 amu for an O-atom addition reaction), the analyte’s original isotopic abundance pattern is preserved in the product ion spectrum. This allows results to be verified from multiple analyte isotopes, and also enables isotope-based measurements such as isotope ratio and isotope dilution to be used (7).
In this article, we compare the performance of MS-MS mode and single-quadrupole (SQ) mode (comparable to conventional quadrupole ICP-MS) for Ti analysis. Using both O2 and NH3 as reaction gases, we investigate Ti measurement in the presence of potential interferences from simple, single-element matrices, and complex matrices.
Experimental Instrumentation
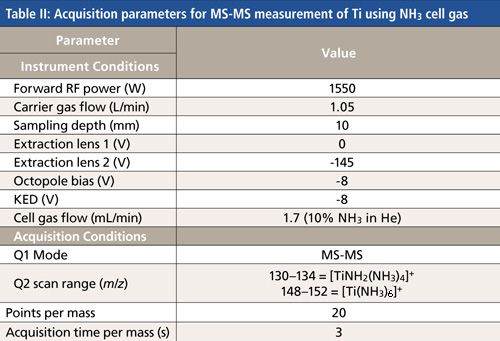
A triple-quadrupole ICP-MS system (Agilent Technologies model 8800 ICP-QQQ) was used for all measurements. The instrument configuration and operating modes have been described previously (8). The standard configuration with Micromist glass concentric nebulizer, quartz sample introduction system, and Ni interface cones was used. Operating conditions are shown in Tables I and II.
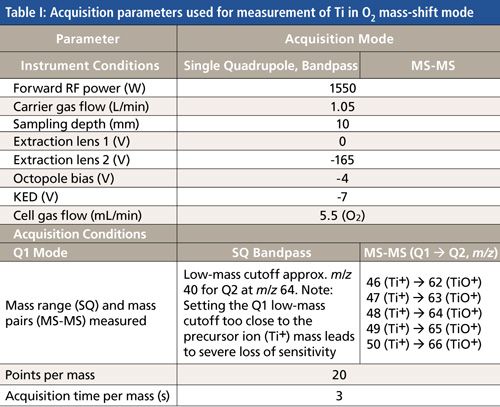
Calibration Standards and Sample Preparation
The standards used for this work were prepared using single-element stock solutions (Spex CertiPrep, Claritas grade). For the TiO+ experiment, the acids were UpA ultrapure grade (Merck).
For the biological matrix experiment, a 0.5-mL sample was diluted with 2.5 mL of diluent mix (0.01% EDTA, Sigma Aldrich 99.999%); 0.005% Triton X-100 (Romil SpA); 4% BuOH (Merck Uvasolv); 1% NH4OH (Fluka TraceSELECT Ultra), then made up to a final volume of 5 mL with ultrapure water (Merck Millipore Element).
Analytical Method
Both O2 and NH3 are suitable cell gases for the analysis of Ti, as both gases react quickly and form product ions with Ti+. O2 cell gas offers simpler methodology, as the product ion measured is TiO+, whereas NH3 cell gas promotes complex, sequential reaction chemistry, leading to a large number of Ti-NH cluster ions. NH3 cell gas therefore has greater potential to generate overlapping product ions from other isotopes and elements present in the extracted ion beam. However, NH3 reaction mode is more selective for Ti, so, for low level analysis in natural sample matrices, NH3 has been shown to provide more effective interference removal (2).
Triple-quadrupole ICP-MS can be operated in MS-MS mode, where both Q1 (before the CRC) and Q2 (after the CRC) operate as unit mass filters. Alternatively, Q1 can be operated as a simple ion guide, or as a bandpass filter, where it passes a range of masses either side of the Q2 set mass. This mode emulates the performance of a conventional quadrupole ICP-MS instrument with a cell that incorporates a bandpass filter. When a mass shift method is used on an ICP-QMS with a “bandpass” cell, the bandpass window must be set to allow both the analyte precursor ion (Ti+ in this case) and the product ion (TiO+ or the Ti-NH3+ cluster ion) to pass through. Thus, the cell has limited ability to reject other ions that might overlap the analyte product ion.
Two experiments were performed using O2 and NH3 cell gas to investigate different aspects of reaction chemistry and the application of MS-MS to avoid error because of ions other than the target analyte ion being present at the analyte mass:
- O2 cell gas: Four 1 ppb Ti standards were prepared, and separate spikes of the elements Ni, Cu, and Zn (at 10 ppb) were added to three of the standards. All four solutions were measured using O2 cell gas with the triple-quadrupole ICP-MS system in both SQ bandpass and MS-MS mode, to investigate whether MS-MS mode could control the interference from other coexisting ions (matrix or other analytes) that might overlap the TiO+ analyte product ions;
- NH3 cell gas: Ti was measured in a biological reference material, using the triple-quadrupole ICP-MS system with ammonia reaction gas, to assess whether MS-MS mode gave control of the reaction chemistry such that a single ammonia-adduct was consistently added to each Ti ion, retaining the isotopic pattern for Ti, and avoiding overlap from other Ti-NH3 cluster ions and any other matrix-based NH3 clusters.
Results
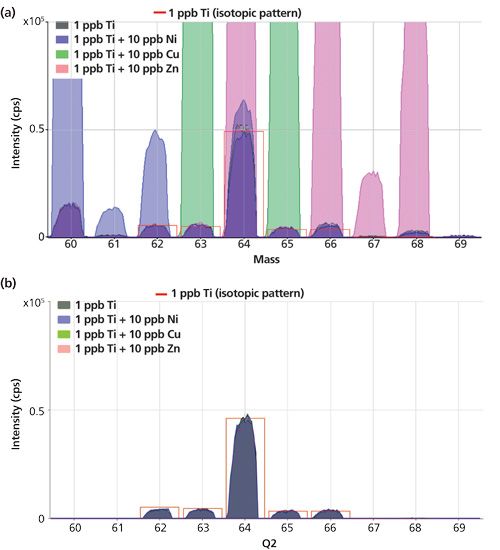
The results for the spiked and unspiked Ti standards measured with O2 cell gas are presented as overlaid spectra in Figures 1a and 1b; the color of the spectrum indicates which spike element gave rise to the peaks. Figure 1a shows the spectra measured in SQ bandpass mode, indicating that the coexisting Ni, Cu, and Zn ions remained at their original masses, and so gave significant overlaps on all the Ti16O+ product ion isotopes. This demonstrates that a bandpass cell cannot exclude other ions that lie within the mass range between the precursor and product ions of the target analyte. In some cases, the overlapping ions may also react with the chosen cell gas, and thereby be moved away from the analyte product ion mass, but that cannot always be relied upon (as in the case of Ni, Cu, and Zn with O2 cell gas). By contrast, the overlaid spectra in Figure 1b demonstrate that, in MS-MS mode, the coexisting Ni, Cu, and Zn ions were all rejected by Q1 (which was set to the appropriate Ti+ precursor mass). As a result, consistent measurement of all five Ti isotopes in all the spiked standards was possible, with the measured peaks matching the natural isotopic abundances of the Ti isotopes.
The recovery data for the biological matrix reference material (Seronorm Trace Elements Whole Blood L-1) are shown in Table III. Note that the spike levels in this reference material (Lot MR4206) are extremely low compared to many other similar reference materials. The blood CRM was measured using a standard addition calibration (spikes at 1 µg/L and 5 µg/L, prepared in the sample diluent matrix). In MS-MS mode, accurate recovery of the reference value was achieved at the sub-microgram-per-liter (single nanomole-per-liter) level for all five Ti isotopes measured as their [TiNH2(NH3)4]+ product ions, demonstrating the specific reaction chemistry capability of MS-MS mode. The [Ti(NH3)6]+ product ion also gave reasonable recovery (a mean of 100% across the Ti isotopes), although the variation was greater because of the lower counts for this product ion.
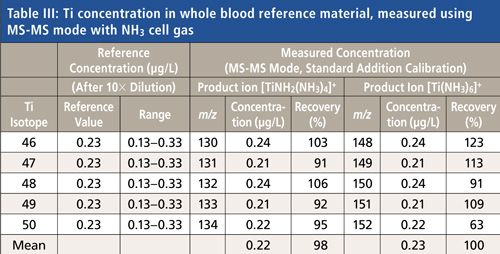
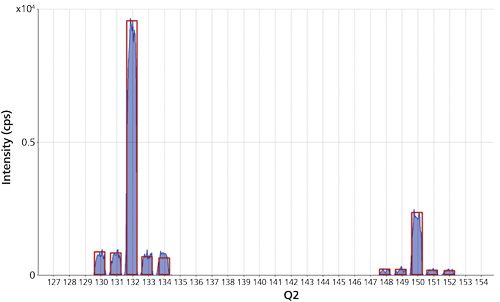
Figure 2 shows a spectrum of the 1 µg/L standard addition spike in the blood reference material; both groups of Ti-NH3 product ions match the natural Ti isotopic abundance template, demonstrating how MS-MS allows specific reaction transitions to be measured, without any other coexisting elements (or isotopes) contributing to the target product ion.
Conclusions
The use of MS-MS mode with triple-quadrupole ICP-MS allows complex reaction chemistry to be controlled, providing consistent measurement of all Ti isotopes in the presence of coexisting analyte or matrix elements, using O2 or NH3 cell gas.
Reaction chemistry can be used in the collision–reaction cell of a conventional quadrupole ICP-MS system to address some polyatomic and isobaric interferences. However, mass selection before the cell is not possible, so other ions present in the sample or derived from the solvent or the plasma (Ar, O, N, H, and so on) can enter the cell and cause overlaps on the target analyte ions, either as preexisting, or cell-formed product ions. Note that the triple-quadrupole ICPMS system is not intended to be used for diagnostic procedures.
References
(1) United States Centers for Disease Control and Prevention, Current Intelligence Bulletin 63, Occupational Exposure to Titanium Dioxide, DHHS (NIOSH) Publication No. 2011-160 (2011).
(2) L. Balcaen, E. Bolea-Fernandez, M. Resano, and F. Vanhaecke, Anal. Chim. Acta. 809, 1–8 (2014).
(3) E. McCurdy and G. Woods, J. Anal. At. Spectrom. 19, 607–615 (2004).
(4) B. Hattendorf and D. Gunther, J. Anal. At. Spectrom. 15, 1125–1131, (2000).
(5) J. Olesik and D. Jones, J. Anal. At. Spectrom. 21, 141–159 (2006).
(6) R. Thomas, D. Price, C. Sieniawska, L. Jung, and F. Abou-Shakra, Spectroscopy 28(11), 28–34 (2013)
(7) L. Balcaen, G. Woods, M. Resano, and F. Vanhaecke, J. Anal. At. Spectrom. 28, 33–39 (2012).
(8) E. McCurdy and G. Woods, Spectroscopy 27(10), 18–29 (2012).
Ed McCurdy is an ICP-MS Product Manager and Glenn Woods is an ICPMS Applications Specialist at Agilent Technologies LDA (UK) Ltd., in Cheshire, UK.
Direct correspondence to: ed_mccurdy@agilent.com

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.