Ultralow Detection of Estrogenic Compounds by GC–NCI-MS-MS
Special Issues
A number of clinical situations now call for high-sensitivity measurement of estrogens, including monitoring during female hormone replacement therapy, antiestrogen treatment, and estrogen deficiency in men. Traditional immunoassay methods and liquid chromatography–tandem mass spectrometry (LC–MS-MS) do not provide the sensitivity and selectivity required for these applications. In contrast, a gas chromatography–negative chemical ionization–tandem mass spectrometry (GC–NCI-MS-MS) platform can provide detection limits below 1 pg/mL when used in conjunction with the appropriate derivatization protocol, with very short cycle times.
Estrogens play a key role in developing and maintaining the female phenotype, germ cell maturation, and pregnancy. Processes that are not gender specific, such as overall body growth, nervous system maturation, bone metabolism/remodeling, and endothelial responsiveness are also mediated by estrogenic compounds.. Although present in both genders, estrogen concentrations are significantly higher in females. Estrone (E1) and estradiol (E2) are the two major biologically active estrogens in nonpregnant humans, while a third bioactive estrogen, estriol (E3), is the main pregnancy estrogen (1). The clinical determination of serum estrogen levels often is used to monitor low-dose female hormone replacement therapy (HRT) and assess fracture risk in postmenopausal women. In premenopausal women, estrogen levels can be monitored to assess ovarian status, including follicle development, for assisted reproduction protocols such as in vitro fertilization. Estrogens also play a role in precocious as well as delayed puberty in females, and can be monitored as part of estrogen replacement therapy in hypogonadal premenopausal women, in conjunction with luteinizing hormone (LH) measurements. Estrogen level measurements are taken as well during aromatase inhibitor treatment of hormone-dependent breast cancer.
Some of the metabolites of the estrogens have been implicated in disease development, such as increased systolic blood pressure in both males and females. Because the metabolites can have a longer half life than the estrogens themselves, there is interest in using them as biomarkers of disease. There is a clear association between excessive exposure to estrogens and the development of cancer in hormone-sensitive tissue such as breast and endometrium (2). The estrogen quinone metabolite, E2-3,4-quinone, has been identified as a potential risk factor, and a subset of women with particular cytochrome P450 enzyme haplotypes have an increased risk of breast cancer as a result (3). The catechol metabolites of estrogens are considered intermediates in estrogen-induced carcinogenesis and their mode of action might be to induce deoxyribonucleoside adducts (4,5). Estradiol is a potent preventative against oxidative neurodegenerative disease by virtue of the hyperoxide-dependent hydroxylation of E2 to its catechol metabolites. In addition, E2 and its metabolites 2-hydroxyestrone (2-OHE1) and 16 a-hydroxyestrone (16-OHE1) also are believed to curtail oxidative stress that can lead to disease conditions including atherosclerosis (6,7). Utilization of the estrogen metabolites as biomarkers will require reliable detection at ultra-low concentrations.
Sensitive, Selective Methods are Needed
Distinguishing serum E2 levels within the low postmenopausal range of <1 pg/mL to 30 pg/mL is a valuable tool in the study of common diseases of older women such as osteoporosis, cognitive dysfunction, cardiovascular disease, and breast cancer (8). Ultralow estrogen measurements also are needed to diagnose inborn errors of sex steroid metabolism, disorders of puberty, and estrogen deficiency in men. Reaching the lowest levels also might be important in predicting risk of fractures (9) as well as monitoring response to antiestrogens for prevention of breast cancer (10) and degree of E2 suppression in women receiving aromatase inhibitor therapy (11,12).
During second-line treatment of hormone-dependent breast cancer, basal and suppressed serum E2 levels are monitored in women receiving aromatase inhibitor therapy. However, assays for E2 were developed originally for younger women — that is, with a range of interest higher than 50 pg/mL. At decreased postmenopausal levels, these assays have varied in accuracy and have lacked standardization. Moreover, aromatase inhibitor therapy requires measurement of E2 at an order of magnitude lower than normal basal levels in postmenopausal women. Ultrasensitive cell bioassays can reach the required levels but are difficult to employ, not widely available, and are not completely specific. Isotopic kinetic methods have been used to determine the degree of E2 suppression but are not practical for clinical use (12).
Direct and indirect immunoassays have been employed widely in clinical settings to measure E2. Indirect assays such as RIAs require an initial extraction step, whereas direct assays do not require extraction. However, both types of immunoassays have overestimated E2 concentrations in postmenopausal women versus mass spectral methods, and are less reproducible.
Liquid chromatography–electrospray ionization tandem mass spectrometry (LC–ESI-MS-MS) methods have been developed to measure estrogens. ESI typically offers one the ability to analyze a compound, or panel of compounds, without the need for derivatization techniques commonly used in gas chromatography (GC)–MS applications. However, for an LC–MS-MS method to approach the detection limits described herein, derivatization is required. One such report states a detection limit of 1 pg/mL of dansyl derivatized E2 in serum (13). GC–MS-MS methods in the electron ionization (EI) mode also have been developed, but again do not have the sensitivity required for these applications.
GC–MS-MS in NCI Mode Delivers Selectivity and Ultralow Detection
The power of negative chemical ionization (NCI) to eliminate chemical interferences provides the ability to quantify therapeutic and subtherapeutic levels of estrogens in complex matrices, when utilized on a GC–MS-MS platform (14,15). These assays typically provide much lower limits of quantification than the LC–MS-MS counterparts, which do not have the sensitivity required for these applications.
Assays for estrogens performed on a GC–NCI-MS-MS platform usually require isolation of the analytes by solid-phase extraction (SPE) followed by derivatization. With automated extraction procedures and advancements in derivatization techniques, GC analysis has become competitive with LC sample preparation and sample analysis cycle times.
Operation in NCI mode using halogenated derivatives allows for electron-capture processes to take place. Compared with EI mode, there is less fragmentation of the molecular ion, hence the term, "soft ionization." The result is a high degree of selectivity and sensitivity, with significantly improved detection limits. In NCI mode, selectivity for the analyte is higher relative to that for the matrix and other common interfering ions observed in EI or positive chemical ionization (PCI) modes. The result is less chemical noise, which translates directly into improved detection limits.
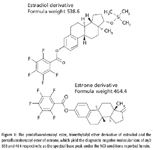
Figure 1
Therefore, the key to sensitive detection when using GC–MS-MS in NCI mode is the optimal derivatization moiety. The pentafluorobenzoyl ester derivatives of estrogens have been shown to be stable and provide diagnostic negative molecular ions (16). These derivatives have proven to be superior to trifluoroacetyl, heptafluorobutyl, pentadecafluorooctanoyl, and perfluorotolyl derivatives (17).

Table I: Regression analysis for estradiol and estrone standard curves*
Sensitive, Precise, and Accurate Quantification of Estrogens
An estrogen-specific assay has been developed on the GC–NCI-MS-MS that can detect as little as 0.15 pg/mL of estradiol reliably. Derivatization of the estrogens was carried out using pentafluorobenzoyl chloride and N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA), which resulted in the formation of the benzoyl ester for the phenol groups, and the trimethylsilyl (TMS) ether for the 17-hydroxyl group of estradiol. Figure 1 shows the structures of the resulting derivatives of estradiol and estrone. The GC method analysis time is approximately 5 min and the cycle time is approximately 6 min.

Figure 2
The assay is repeatable as well as reproducible. Nearly 100 injections were made without the need for any routine maintenance. The signal-to-noise ratio (S/N) is greater than 10 and chemical interferences that could affect quantification are not detected. The assay is linear from 0.3 to 80 pg/mL of estrone and 0.75 to 200 pg/mL of estradiol, giving an R2 > 0.996 for both E2 and E1. Precision (%CV) and bias (% difference from expected) are less than 15% across the quantification range for both analytes as well (Table I). Three concentrations of serum QC samples were prepared and analyzed that bracketed the quantification range of the assay for both estrogens. Precision and bias are less than 11% (accuracy >89%) at all concentrations for estradiol, and less than 15% for all concentrations of estrone (Table II). Figure 2 illustrates the ease of detection at the lowest limit of detection (LLOD) for estradiol, 0.15 pg/mL (S/N = 41), as well as the lowest limit of quantification (LLOQ), 0.3 pg/mL. The LLOQ for estrone using this assay is 0.8 pg/mL (Figure 3).
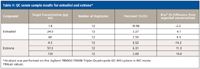
Table II: QC serum sample results for estradiol and estrone*
Conclusion
There is a current and growing need to detect very low levels of estrogens and their catechol metabolites reliably. Traditional immunoassays cannot provide the selectivity needed, and LC–MS-MS systems, the current industry standard for bioanalytical analysis, fall short in the ability to chromatographically separate interferences and provide the sensitivity required to quantify levels of certain endogenous compounds. Analysis by GC–NCI-MS-MS has been the primary analytical tool for monitoring endogenous levels of analytes considered to be biomarkers. The selectivity of the derivative, the high performance separation of GC, the response of the halogenated derivatives in NCI mode, and the elimination of matrix interference by SRM analysis provide the needed performance in the development of a biomarker assay.

Figure 3
There also have been significant advancements in the past few years in GC–MS-MS technology that maximize ion transmission and detection, and the use of fast GC would further reduce runtime. When the ultimate level of detection and quantification is required to measure an ultra-low level endogenous compound, then a GC–NCI-MS-MS system might be the best alternative.
Anthony Macherone and Melissa Churley are application scientists with Agilent Technologies, Santa Clara, California. Robert White is with Taylor Technology, Inc., Princeton, New Jersey.
References
(1) Mayo Medical Laboratories web site, accessed 3/2/2010.
URL: http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/81816
(2) J. L. Bolton and G. R. Thatcher, Chem. Res. Toxcol. 208, 93–101 (2008).
(3) F. F. Parl et al., Ann. NY Acad. Sci. 1155, 68–75 (2009).
(4) E. Yagi et al., Carcinogenesis 22, 1505–1510 (2001).
(5) D. E. Stack et al., Chem. Res. Toxcol. 9, 851–859 (1996).
(6) J. Nilsen, Front Neurodenocrinol. 29, 463–475 (2008).
(7) M. Sowers et al., Clin. Endocrinol. 68, 806–813 (2008).
(8) J.S. Lee et al., J. Clin. Endocrinol. Metab. 91, 3791–3797 (2006).
(9) S.R. Cummings et al., New Eng. J. Med. 339, 733–738 (1998).
(10) S.R. Cummings et al., J. Amer. Med. Assoc. 287, 216–220 (2002).
(11) J. Geisler et al., J. Clin. Oncol. 20, 751–757 (2002).
(12) L. M. Demers, Steroids 73, 1333–1338 (2007).
(13) S.C. Tai and M.J. Welch, Anal. Chem. 77, 6539–6563 (2005).
(14) R.S. Santen et al., Steroids 72, 666–671 (2007).
(15) X. Xu et al., Anal. Chem. 79, 7813–7821 (2007).
(16) S. Nakamura et al., J. Chromatogr., A 275–282 (2001).
(17) X.-Y. Xiao and D. McCalley, Rapid Commun. Mass Spectrom. 14, 1991–2001 (2000).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.