1064-nm Raman: The Right Choice for Biological Samples?
Special Issues
Interference from background fluorescence is a common challenge in Raman analysis. A study of three different types of biological samples was made to compare the ability of 785-nm and 1064-nm excitation to deal with this problem.
Near-infrared (NIR) excitation is often touted as a method of reducing background fluorescence in organic samples, but at considerable sacrifice to signal strength. Is the tradeoff worth it? Here we compare data from bovine bone, monosodium urate crystals isolated from synovial fluid, and demineralized tooth enamel using 785- and 1064-nm Raman spectroscopy to assess factors relevant to data quality. The results indicate that 1064-nm Raman vastly outperforms 785-nm Raman for background fluorescence reduction, even as compared to lengthy sample pretreatment at 785 nm via photobleaching. As cost-effective, sensitive systems for 1064-nm Raman spectroscopy become more widely available, it has become a viable technology for the measurement of a diverse range of biological samples to minimize sample damage and optimize the quality of spectral data.
Interference from background fluorescence is a common challenge in Raman spectroscopic analysis of organic and biological specimens (1–6). It can degrade the signal-to-noise ratio of the data collected, and in some cases obscures the Raman spectrum entirely. The chemical complexity of biological samples, in particular, presents many possible sources of autofluorescence, from aromatic groups like tryptophan, tyrosine, and phenylalanine in folded proteins (7) to very intense carotenoid bands in blood that interfere in hemoglobin studies (8).
Although fluorescence can be dealt with via a variety of techniques such as the use of confocal configuration, photobleaching, or chemical bleaching (9), the most effective method of removing or reducing fluorescence has been through excitation at longer wavelengths. While this diminishes the background fluorescence burden, it results in much lower signal intensity (scaling inversely with the fourth order of the excitation wavelength). The use of near-infrared (NIR) excitation at 785 nm has thus been the standard approach in addressing background fluorescence for organic materials because it provides a reasonable compromise between signal level and background fluorescence.
Photobleaching of samples may be used in tandem with, or in lieu of, 785-nm excitation for fluorescence reduction in biological samples. Though the mechanism is not well understood, prolonged exposure to excitation light often results in a decrease in autofluorescence. Golcuk and colleagues (10) demonstrated the collection of Raman signal from bone tissue with 532-nm laser excitation after samples were subjected to 30–120 min of photobleaching. Even when a reasonable Raman spectrum can be acquired, the signal-to-noise ratio may be improved by using brief photobleaching, as shown in a study of monosodium urate (MSU) crystals isolated from joint fluids (5).
Similarly, chemical bleaching with agents such as peroxides can reduce interference from fluorescence in some tissues, but the process can take up to several hours and may impact the spectral profile and band ratios (9). It must also be halted in a very narrow window to avoid damaging tissue, such as bone (11). Sample damage and increased data acquisition time are the key limitations associated with both photobleaching and chemical bleaching, particularly in clinical settings.
Although 785-nm excitation is largely indicated for Raman analysis of biological or organic analytes (12,13), there are circumstances in which such systems fail to acquire adequate Raman spectra because of high fluorescence, as seen with lung (14), liver, and kidney tissues (15). These samples are better served with 1064-nm excitation, as are plant samples studied or classified through the analysis of lignins (16,17).
The use of 1064-nm excitation results in a dramatic decrease in background for highly fluorescing samples such as biological tissues, edible oils, petrochemicals, polymers, and many inks and pigments. Fourier transform (FT) Raman systems based on 1064-nm excitation have existed for more than two decades, but typically require integration times in excess of 30 min (8,18,19). Dispersive 1064-nm Raman systems provide an alternative, but have historically been limited by the high cost and relatively poor signal-to-noise ratio of cooled InGaAs detector arrays. The emergence of more affordable, higher performance detectors has enabled a burst of commercial dispersive 1064-nm Raman systems in recent years, spurring a variety of new studies (15–17). Acquisition times in dispersive Raman systems can still be moderately long, often 30 s or more, unless a high-throughput 1064-nm system is used. This contributes to noise and can limit applicability, underscoring the need to select a dispersive 1064-nm Raman system carefully.
Although many individual studies have been undertaken to determine the optimum excitation wavelength for a given sample, few systematic comparisons of 785-nm versus 1064-nm excitation in biological samples have been carried out. The goal of this study was to determine whether 1064-nm excitation presents any significant merits in reducing fluorescence background relative to 785-nm excitation. This knowledge is essential to potential buyers in the market to determine whether there is added value in choosing a dispersive 1064-nm system for biological studies.
In addition, the uncharted dynamics of photobleaching at 1064-nm excitation in these systems is explored here. The detailed comparison of fluorescence recorded from the same sample set using 785-nm and 1064-nm dispersive Raman systems included analysis of the reduction in fluorescence relative to the duration of photobleaching.
Results collected from three biological samples showed significantly lower fluorescence background in Raman spectra collected with 1064-nm excitation as compared to excitation at 785 nm. Furthermore, fluorescence background was found to be more stable with 1064-nm excitation, such that the background reduction with photobleaching was minimal. In short, 1064-nm excitation presented significant merits compared to 785 nm in dealing with background fluorescence.
Experimental
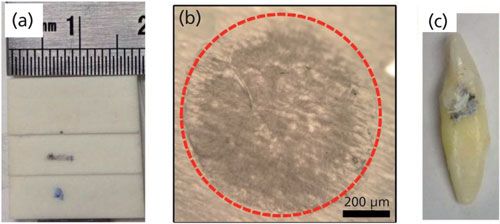
Three different types of biological specimens were used in this study: a slice of bovine bone, a sample of monosodium urate crystal that was isolated from human joint fluid (as described in reference 5), and a human tooth (Figures 1a–1c, respectively). Human tissues were collected under the approvals of the appropriate institutional review boards.
Figure 1: Images of samples: (a) bovine bone slice, (b) monosodium urate (MSU) crystals, and (c) human tooth with lesion.
The systems used for measurement included an integrated 785-nm Raman system and a modular 1064-nm system. The 785-nm Raman system (WP 785L, Wasatch Photonics) included a 785-nm laser and an f/1.3 spectrometer optical design integrated with a charge-coupled device (CCD) array cooled to 15 °C to reduce system noise. Integrated free space sampling optics with a 25-mm focal length was used to deliver the laser light to the sample over a 70-µm spot, as well as to collect the Raman signal.
The 1064-nm system was an early prototype of a commercial modular Raman system with sample coupling via fiber probe. The spectrometer provided a spectral resolution of 10 cm-1 at a 50-µm slit width using a volume phase holographic transmission grating (Wasatch Photonics). It included a TEC-cooled 512-element InGaAs array detector (Du490A-1.7, Andor). Excitation light was provided with an 800-mW, 1064-nm laser (Innovative Photonics Solutions), coupled to a Raman probe with a focal distance of 10 mm (Wasatch Photonics).
The power used for photobleaching was set at 50 mW at 785 nm; the exposure time was 1 s and the spectra were averaged 10 times, resulting in 10 s total integration time. For the 1064-nm system, a 500-mW laser beam was used for photobleaching, and spectra were acquired as a single scan with a 10-s integration time. The sample was positioned with the laser power set at 5% of the photobleaching level before the start, then aligned at the focal point of the system or probe. The first spectrum was acquired as soon as the photobleaching process began; the ensuing spectra were then recorded at intervals as photobleaching proceeded. Noise levels reported were determined using the standard deviation of the data points in a region free of Raman peaks (1150–1200 cm-1 for bovine bone, and 650 cm-1 for MSU crystals).
Results and Discussion
Sample 1: Bovine Bone
Raman microspectroscopy is used in the study of bone tissue for its ability to provide composition information relevant to biomechanical properties and mineralization processes at micrometer-range spatial resolution (5). High fluorescence background in these samples obscures important Raman bands for mineral and matrix components, however, necessitating the use of chemical treatment or photobleaching at 532-nm (10) or 785-nm excitation (13).
The dried bovine bone measured in this study yielded significantly higher background fluorescence when illuminated with 785-nm excitation (Figure 2a, top three curves) than with 1064-nm excitation (Figure 2a, bottom three curves). The 1064-nm Raman spectra all appeared to share a common background, seen also in a spectrum taken of a blank glass microscope slide (Figure 2b, central, orange curve). This was concluded to be the 1064-nm system response because it did not change after 20 min of exposure. Possible sources of this system response include silica Raman background from the fiber probe (20), or more likely stray light because of scattered light in the Raman probe, resulting in refinements to the optical design.
Figure 2: (a) Time-dependent Raman spectra of bovine bone acquired by the 785-nm Raman system (upper set) and the 1064-nm Raman system (lower set). Black, red, and blue curve indicate 0-, 1-, and 15-min photobleaching, respectively. The central, orange curve was the response of the 1064-nm system, in which a blank glass slide was used to acquire the spectrum. (b) Raman spectra before and after system response correction. Accurate system response was unknown; it was arbitrarily estimated to be 50% of the glass system response curve.
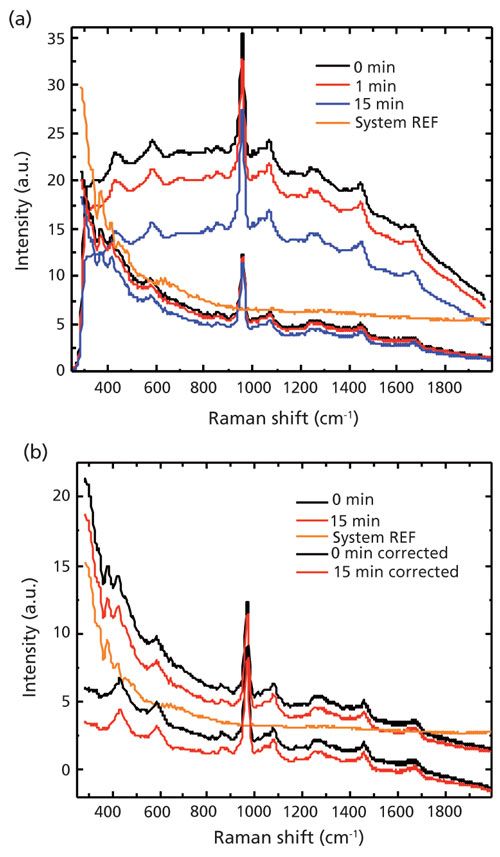
No studies were performed to compare the system response from different samples (which reflect excitation laser back into system); the system response in the case of bovine bone was arbitrarily estimated as 50% of that of glass slides for the purposes of analysis.
Comparing the baseline of the bone data and the system response, it was concluded that the system response contributed largely to the baseline of the bovine bone that was recorded by the 1064-nm system. Taking into account the difference in laser power and the integration time, the fluorescence background seen in spectra from the 785-nm system was more than 500 times (in counts) greater than that from the 1064-nm system before photobleaching. An even higher ratio can be deduced if the baseline contributed from the system response is subtracted, as shown in Figure 2b.
Notwithstanding the initial intensities, the fluorescence level experienced a reduction with photobleaching experiments on bovine bone for both the 785-nm and 1064-nm systems. The rate of photobleaching was much faster at 785 nm, as can be seen in Table I, decreasing by 35% after 15 min versus 15% for 1064 nm.
Sample 2: MSU Crystals
MSU crystals are frequently observed in the joint space, and lead to symptoms of gout. Raman spectroscopy is a promising diagnostic technique to identify these crystals, but it suffers from high background fluorescence (5).
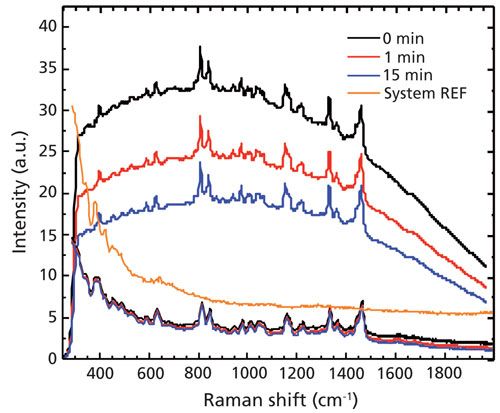
In this study, MSU crystals isolated from synovial fluid and analyzed using Raman spectroscopy presented rapid background reduction in response to photobleaching at 785-nm excitation, while the photobleaching rate in response to the 1064-nm system was low (Figure 3). Total reduction in photobleaching at 1064 nm was only 13% after 15 min, indicating much greater sample stability with light exposure (Table I).
Figure 3: Time-dependent Raman spectra of MSU crystals taken by a 785-nm Raman system (upper set) and 1064-nm Raman system (lower set). The central, orange curve was the response of the 1064-nm system, in which a blank glass slide was used to acquire the spectrum.
Sample 3: Lesions on Teeth
White lesions on human teeth normally indicated demineralized enamel and have higher concentration of organic phases than healthy enamel. In fact, the progression of carious lesions in teeth can be estimated using the high background in their Raman spectra, as has been shown with 785 nm Raman measurements (12).
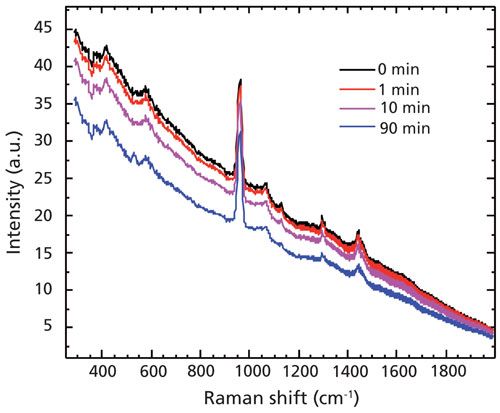
In this study, the tooth had a white lesion on part of the enamel surface, and dark colored spots were present within the white lesion. The white lesion region showed lower mineral intensity but higher fluorescence background (data not shown). Attempts to measure the dark spots within the white lesion with the 785-nm system resulted in intense fluorescence that saturated the system and prevented collection of suitable Raman spectra, even after 1 h of photobleaching at a 0.5-s data integration time. The use of the 1064-nm Raman system, however, resulted in the successful recording of spectra from the same spot (Figure 4). Moderate fluorescence reduction was observed with 1064-nm excitation over time (Table I).
Figure 4: Time-dependent Raman spectra of opaque spot on tooth lesion taken using a modular 1064-nm Raman system.
Conclusion
A direct comparison of fluorescence background in Raman spectroscopic analysis of various biological samples at both 785 nm and 1064 nm showed the longer wavelength to offer significant advantages. Fluorescence background of Raman spectra excited at 1064 nm was more than 500-fold lower than that obtained by 785-nm excitation after differences in excitation energy and acquisition time were taken into account.
Additionally, autofluorescence from several samples was so intense as to saturate the detector during measurements at 785 nm, whereas excitation at 1064 nm enabled Raman analysis with even the most fluorescent spots encountered. Furthermore, the background reduction with photobleaching was minimal with 1064-nm excitation, resulting in a more stable background with sample exposure.
Given the current availability of high quality commercial 1064-nm Raman systems at a reasonable cost, users who are facing a diverse set of biological specimens may therefore see greater value in the balance of data quality, acquisition time, and cost offered by 1064-nm Raman as compared to 785-nm systems. Even taking into account established pretreatment methods, 1064-nm excitation presents significant merits over 785-nm excitation in dealing with background fluorescence.
Acknowledgments
The authors acknowledge Wasatch Photonics Inc., for their assistance in providing the demo Raman units of 1064 nm; as well as Bolan Li and Anna Akkus for providing the samples. This study was funded by the research grant R01AR057812 (OA) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH).
References
- N. Everall et al., J. Raman Spectrosc. 17(5), 415–423 (1986).
- A.R. Masri, R.W. Bilger, and R.W. Dibble, Combust. Flame68(2), 109–119 (1987).
- D.B. Chase, J. Am. Chem. Soc. 108(24), 7485–7488 (1986).
- A.P. Shreve, N.J. Cherepy, and R.A. Mathies, Appl. Spectrosc. 46(4), 707–711 (1992).
- S. Yang et al., J. Raman Spectrosc. 44(8), 1089–1095 (2013).
- Y.K. Min et al., J. Raman Spectrosc. 36(1), 73–76 (2005).
- F.W.J. Teale, and G. Weber, Biochem. J. 65, 476–482 (1957).
- H. Sato et al., J. Biomed. Opt. 6(3), 366–370 (2001).
- T-C. Chen, D.A. Shea, and M.D. Morris, Appl. Spectrosc. 56(8), 1035–1037 (2002).
- K. Golcuk et al., Biochim. Biophys. Acta, Biomembr. 1758(7), 868–873 (2006).
- D.A. Shea and M.D. Morris, Appl. Spectrosc. 56, 182–186 (2002).
- W. Hill and V. Petrou, Appl. Spectrosc. 54(6), 795–799 (2000).
- M.V. Schulmerich et al., J. Biomed. Opt. 13(2), 021108 (2008).
- Y.K. Min et al., J. Raman Spectrosc. 36(1), 73–76 (2005).
- C.A. Lieber, H. Wu, and W. Yang, “Tissue Measurement Using 1064 nm Dspersive Raman Spectroscopy,” presented at SPIE BiOS, International Society for Optics and Photonics, San Francisco, California, 2013.
- M.W. Meyer, J.S. Lupoi, and E.A. Smith, Anal. Chim. Acta706(1), 164–170 (2011).
- J.S. Lupoi and E.A. Smith, Appl. Spectrosc. 66(8), 903–910 (2012).
- T. Hirschfeld and B. Chase, Appl. Spectrosc. 40(2), 133–137 (1986).
- J.J. Baraga, M.S. Feld, and R.P. Rava, Proc. Natl. Acad. Sci. U.S.A. 89(8), 3473–3477 (1992).
- M.L. Myrick, S.M. Angel, and R. Desiderio, Appl. Opt. 29(9), 1333–1344 (1990).
Shan Yang is an Assistant Professor of Physics with the Department of Physics at Jackson State University in Jackson, Mississippi. Ozan Akkus is a professor with the Departments of Mechanical & Aerospace Engineering, Orthopaedics, and Biomedical Engineering at Case Western Reserve University in Cleveland, Ohio. David Creasey is the President of Wasatch Photonics in Durham, North Carolina. Direct correspondence to: dcreasey@wasatchphotonics.com

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.