Advantages and Disadvantages of Different Column Types for Speciation Analysis by LC-ICP-MS
Speciation analysis by LC-ICP-MS has been growing rapidly in popularity and application over the past several years. Not only have people begun looking at different elements and species, but there has also been an increase in the variety of matrices that speciation analysis is being performed on.

Speciation analysis by liquid chromatography–inductively coupled plasma-mass spectrometry (LC–ICP-MS) has been growing rapidly in popularity and application over the past several years. Not only have people begun looking at different elements and species, but there has been an increase in the variety of matrices that speciation analysis is being performed on: various types of environmental, waste, and process waters; both solid and liquid foods; and biological fluids. With this increased interest, more laboratories are offering speciation analysis as service. When deciding upon a speciation method, many factors must be considered.

Kenneth Neubauer
The chromatographic separation of components is based upon competition between the column, mobile phase components, species, and the sample matrix. With the variety of matrices now being analyzed, there are a variety of chromatographic options available. This column will discuss three column types and the relative advantages and disadvantages of each.
Ion-Exhange Versus Reversed-Phase Ion-Pairing Chromatography
Two common types of separation schemes used in chromatography are ion-exchange and reversed-phase ion-pairing chromatography. These schemes differ primarily in the column stationary phase, which affects the way components compete for active sites on the column.
Ion-exchange chromatography involves the interaction of ionic (that is, charged) components in the mobile phase with charged stationary groups on the column packing material. Charged species in the sample compete with mobile phase components for sites on the column. Species with a stronger attraction to column sites than mobile phase components will be retained on the column longer and have longer elution times. Species with a weaker attraction to the column than the mobile phase components will have shorter elution times. This is the mechanism by which separation is achieved and is represented in Figure 1.
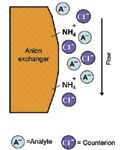
Figure 1: Schematic of the ion-exchange separation mechanism.
Reversed-phase ion-pairing chromatography involves the use of reversed-phase columns that are characterized by nonpolar stationary phases composed of carbon chains, typically 18 carbons (C18), although other columns exist with different length carbon chains (for example, four and eight carbons). Because most inorganic species exist in charged states in solution, it appears that reversed-phase columns would not be able to perform the separation.
This situation is rectified by incorporating an ion-pairing reagent in the mobile phase. Ion-pairing reagents consist of both nonionic and ionic parts: The nonionic sections interact with the carbon chain on the column, and the ionic sections interact with the charged species in solution by mechanisms similar to those in ion-exchange chromatography. In this way, a reversed-phase column can be made to perform like an ion-exchange column. The mechanism of reversed-phase ion-pairing chromatography is shown in Figure 2.
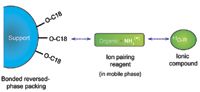
Figure 2: Diagram illustrating the reversed-phase ion-pairing mechanism.
Both ion-exchange and reversed-phase ion-pairing chromatography each have their advantages and disadvantages. One of the main advantages of ion exchange is that there is only one interaction involved in the separation: the analytical species interacting with the stationary phase. With reversed-phase ion pairing, two interactions are involved with the separation: the ion-pairing reagent interacting with stationary phase of the column and the species interacting with the ion-pairing reagent. As a result, ion-exchange chromatography may have more matrix tolerance.
The downside of ion-exchange chromatography is that these columns typically are much more expensive than reversed-phase columns. Also, because reversed-phase columns have been in use for many years, they are reliable and established, meaning that results will be reproducible from one column to the next. This may not always be true with ion-exchange columns because new columns are constantly being developed with new ionic groups bound to the stationary phase. Because of this constant evolution, some ion-exchange columns may not always be well established, which could lead to irreproducibility from column to column.
The decision to use either ion-exhange or reversed-phase ion-pairing chromatography depends upon the application: the elements and species of interest, the sample matrix, and the levels of the different species that need to be measured.
Standard-Bore Versus Narrow-Bore Columns
Regardless of which type of column is chosen, users are then faced with another choice: column diameter. For speciation analysis, the two most common column diameters used are 4.6 mm (referred to as standard bore) and 2.1 mm (narrow bore). Again, each diameter offers specific advantages and limitations.
Standard-bore columns are available in a variety of particle sizes, ranging from 3 μm to 10 μm. Depending upon the size, the optimal high performance liquid chromatography (HPLC) flow rate for achieving the best separation can vary from 0.7 mL/min to 2 mL/min, as shown in the Van Deemter plot in Figure 3. In this figure, the HETP label on the y-axis refers to the height equivalent to a theoretical plate — the lower the HETP, the better the separation. As shown in the Van Deemter plot, the best separations are achieved with 3-μm packings and a flow rate of 1.5–2 mL/min. ICP-MS can handle 1.5-mL/min sample introduction rates without a problem, so the best separations are achieved with a 3-μm packing and an LC flow rate of 1.5 mL/min. These conditions are well suited for standard-bore columns.
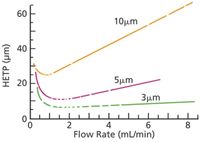
Figure 3: Van Deemter plots for various LC stationary phase particle diameters.
If a 3-μm packing is used in a narrow-bore column, a flow rate of 1.5 mL/min will result in a very high back pressure, typically too high to be run safely. This is the result of the smaller internal diameter of the column. As a result, narrow-bore columns usually are run with LC flow rates of 0.2–0.5 mL/min. As seen in the Van Deemter plot, these low flow rates do not provide the best separation, which means that using a narrow-bore column may involve sacrificing some separation capability. This compromise may or may not be a problem, depending upon the spacing between peaks. However, this usually can be compensated for by changing the mobile phase conditions.
Narrow-bore columns offer two advantages compared with standard-bore columns: sharper peaks and less reagent consumption. The sharper peaks are a direct result of the narrower column width — there is less radial dispersion, which leads to sharper, more intense peaks than those obtained using standard-bore columns. The increased peak sharpness may compensate for the loss of resolution resulting from the nonoptimal flow rates. The increased peak height may also mean that lower levels of the species can be seen.
A main drawback of narrow-bore columns versus standard-bore columns is that their capacity is reduced. Because fewer stationary phase particles can physically fit into the narrower column, there is less surface area available for species to bind. As a result, these columns can become overloaded easily, which could be a problem when analyzing unknowns.
Typically, this reduced capacity is compensated for by diluting the samples more or using smaller injection volumes. Because less sample is injected onto the column, this may offset the advantage of increased peak height. Therefore, it is not always possible to measure lower levels with a narrow-bore column.
Another potential disadvantage of narrow-bore versus standard-bore columns is sample throughput. Because lower LC flow rates are used for narrow-bore columns, separations tend to take longer than with standard-bore columns, which will affect sample throughput. The advantage of a lower LC flow rate is that there is less consumption of reagents and less waste to dispose.
When deciding whether to use a standard-bore or narrow-bore column, users must consider which combination of factors is best for a particular laboratory and application. There is no single correct answer — each situation is different.
HPLC Versus UHPLC for Speciation
Within the past several years, a new form of LC has emerged: ultrahigh-pressure liquid chromatography (UHPLC). The Van Deemter plot shows that the smaller the particle size in the column, the better the separation. UHPLC uses columns with particle sizes less than 3 μm, typically 1.5–2.5 μm. These smaller particles provide more surface area for mobile phase and species to interact, which leads to better separation. Or, another way to think of it is that shorter columns with smaller packings can provide the same separation as longer columns with larger packings. The advantage of the shorter columns is that the time for separations are reduced greatly.
The other signifcance of the smaller packings is a much higher back pressure, typically more than 10,000 psi. This increased pressure requires a special HPLC pump capable of handling it. Normal HPLC pumps cannot be used with columns containing packing material with a diameter of <3 μm, hence, the development of UHPLC.
The question now becomes: Can UHPLC provide an advantage for speciation analysis? At first glance, the obvious answer would be "Yes." However, when other aspects of UHPLC are considered, the answer is not so clear-cut. The driving force behind UHPLC development was to shorten the analysis times required for the separation of biological molecules (such as proteins, peptides, and so forth), which can take up to an hour or more. Most typical speciation analyses with either standard- or narrow-bore columns can be accomplished in less than 10 min. Therefore, there would not be a great time savings.
Users of UHPLC also must be aware of other factors associated with these columns. First, because the particles are so small and packed so tightly, the columns can clog easily. To eliminate particulates, all mobile phases and samples must be filtered before analysis. Because ICP-MS is such a sensitive detection method, filtering can introduce conatmination, that could raise the baseline. Also, filtering samples may facilitate the interconversion of species in solution. Therefore, the effects of filtering on species conversion must be studied and understood.
The additional active sites available on UHPLC columns provide the increased separation capability, but this also means that these columns take longer to equilibrate. While a typical 3-μm column may take 20 min to equilibrate, a UHPLC column could take upwards of 45 min for proper equilibration. This increased time would have consequences when starting the system, changing mobile phases, running gradient methods, and performing method development.
Another aspect to consider are the types of UHPLC columns available. Because UHPLC is relatively new and was developed primarily for separation of biological substances, columns that will separate inorganic species of interest may not be available. For example, there are few ion-exchange UHPLC columns available.
Where UHPLC could be advantageous is in the emerging field of metallomics. This field involves the separation of biological substances using ICP-MS to detect inorganic molecules associated with proteins, peptides, and other biomolecules. UHPLC columns may be available to separate the substances of interest.
Summary
When performing speciation analysis, there are many aspects that must be considered: separation scheme, column type, column diameter, mobile phase, species of interest, required measurement levels of each species, sample throughput, and reagent usage and disposal. There is no single right answer for every situation. Each user must consider these factors when undertaking speciation analysis.
Kenneth Neubauer is a Senior Scientist at PerkinElmer LAS, where he works with both ICP-MS and HPLC–ICP-MS. He received a B.A. in Chemistry from Colgate University, Hamilton, New York, and a Ph.D. in Analytical Chemistry from the University of Delaware, Newark. Ken joined PerkinElmer in 1997.

Applications of Micro X-Ray Fluorescence Spectroscopy in Food and Agricultural Products
January 25th 2025In recent years, advances in X-ray optics and detectors have enabled the commercialization of laboratory μXRF spectrometers with spot sizes of ~3 to 30 μm that are suitable for routine imaging of element localization, which was previously only available with scanning electron microscopy (SEM-EDS). This new technique opens a variety of new μXRF applications in the food and agricultural sciences, which have the potential to provide researchers with valuable data that can enhance food safety, improve product consistency, and refine our understanding of the mechanisms of elemental uptake and homeostasis in agricultural crops. This month’s column takes a more detailed look at some of those application areas.