Pressure and Vacuum: Not Really Trivial
Good vacuum system design is a crucial underpinning for high performance instrumentation. The important aspects of pressure and vacuum need regular teaching, and here Ken Busch discusses them.

In the ideal (off) world, we would assemble and operate our mass spectrometers in geosynchronous orbit. With ultralow pressure and infinite pumping capacity right outside the laboratory window, a few solar panels providing the power needed to operate the instrument (which is really minimal outside the power needed to operate vacuum pumps), and no terrestrial distractions, the only remaining impediment would be the wait that may be needed for appearance of a service engineer. The constrained transport chain for getting samples from Earth to the orbiting instrument might prove to be a blessing in disguise, serving to discourage submission of casual or repetitive samples, and focusing attention on properly prepared and validated samples. Should the return visit to Earth for the annual ASMS meeting become problematic, ASMS webcasts could prove useful.

Kenneth L. Busch
Such a scenario may be premature but it is not unrealistic. The location of mass spectrometers off-planet is not limited to the Viking instrument resident on the surface of Mars. Mass spectrometers have sampled the composition of other planetary atmospheres, comets, and interplanetary space itself. The need to design such instruments to meet the constraints of space and power, and to ensure robustness, has informed the development of the newest generation of mobile mass spectrometers for the rest of us. As with all design that pushes to extremes, it is a clear understanding of the basics that catalyzes progress. Note that the basic studies in extraterrestrial mass spectrometry (MS) extend back to the 1950s and 1960s. The designers of such instruments also had unique approaches to creating and maintaining a vacuum in the instrument. Creating a vacuum may be as simple as opening a port to space in transit. Maintaining a vacuum during descent through a planetary atmosphere requires careful consideration of the pressures that may be encountered, the composition of the gases encountered (these were in fact what was to be measured), and gas conductances within the system and through its ports. Perhaps the design would consider a control parameter involving pressure measurements taken on-site, with the readings fed into a system that makes decisions on the fly. These complex topics deserve a more detailed exposition, which will appear in this column eventually. Until then, and to return to Earth orbit, interested readers might learn about the Wake Shield Facility (described at http://www.svec.uh.edu/wsfp.html), which takes advantage of the excellent vacuum available in the wake of the space shuttle.
Earlier columns in "Mass Spectrometry Forum" covered general topics of vacuum systems (as well as our sometimes confusing uses of the terms vacuum and pressure), and the operation of high vacuum pumps (1,2). Here, to keep things simple, we will call any pressure below 1 atmosphere (760 Torr) a vacuum. There are subsidiary terms of rough vacuum, low vacuum, high vacuum, and ultrahigh vacuum, with such terms corresponding, in order, to lower and lower pressures. The pressure in interstellar space, by the way, is about 10-16 Torr, and the pressure within interplanetary space higher (depending upon where you are). These are isotropic gas pressures, and the fact that the interstellar pressure is so low is one reason why radiation pressure can be used to exert a force upon solar sails.
The focus of this column is the measurement of pressure in a mass spectrometer, located somewhere on the surface of planet Earth (±5 km) (3). The continued growth and diversification of MS should refocus our attention on the attainment of vacuum and the accurate measurement of pressures. At the heart of MS is the ability to create ions, to move them around, to differentiate them by their mass-to-charge ratios, and to detect them. For years, and certainly through the dominant era for electron ionization (EI) and chemical ionization (CI) sources, we thought of the MS instrument as under high vacuum from source through to the detector. We also came to know the vacuum pumping system as a high-cost, high-maintenance part of the instrument. Certainly, pumping systems evolved from the crude apparatus first used by Aston in his parabola mass spectrographs, but the consistent general model was a high-vacuum diffusion pump (or two) for the main system to achieve a high vacuum, with associated backing pumps (the rough pumps) that also could be plumbed into source interfaces for separators or direct insertion probes. Most pumps (including diffusion pumps and rotary vane backing pumps) transport gas molecules against a pressure gradient, so the ultimate exhaust for the backing pumps should be a hood vented to outside. The advent of reliable turbomolecular pumps did not shift the basic design of the pumping systems in our instruments, but added certain advantages of speed and pump placement. The basic goal still was to move the gas molecules from inside the system to outside of it.
Within this general scheme, much of the plumbing intricacy of the vacuum pumping system was designed for the ionization source of the mass spectrometer, and the need to transport samples from the outside world (760 Torr) into the mass spectrometer (where the analyzer is at 10-6 Torr). For example, a direct insertion probe would be fitted with a staged series of chambers, valves, and locks so that discrete samples at the laboratory pressure could be introduced safely into the 10-5 Torr EI source, or the 1 Torr CI source. Jet separators and membrane interfaces, and various other devices, were early interface devices that transported a stream of sample molecules from the exit of a gas chromatography (GC) system to those same under-vacuum sources, while pumping away most of the carrier gas and thereby enriching the concentration of sample. For liquid chromatography (LC), an even wider diversity of interface systems was devised because the evaporation of the LC solvent placed an even greater load on the vacuum-pumping system, and in the beginning at least, the ionization source was still operating at a pressure far below atmospheric pressure. Now, ionization sources operate at pressures above atmospheric pressure, and commonly at ambient pressure, such as electrospray ionization (ESI) sources, along with ambient sampling methods, and the problems of designing a successful interface are somewhat eased. At the other extreme, sources in surface science experiments may operate at pressures as low as 10-9 Torr, and the idea of a chemical ionization reagent gas at 1 Torr pressure is anathema. Mass analyzers, however, still usually operate within the pressure range of 10-5 to 10-7 Torr, with the notable exception of the ion trap.
Instrument operators have to be aware of what pressure regime is proper for each part of a more complicated instrument, and measure and monitor those pressures for optimum and stable instrument operation. A rule-of-thumb in practical troubleshooting for erratic or deteriorating instrument operation is to "check the vacuum first." The reason is explained directly in a short commercial publication about the need for accuracy in low pressure measurements (4): "But when it comes to high vacuum measurement, accuracy seems not to matter. We speak of being in "the 6 range" or in "the low part of the 8 range." We seem to ignore the fact that the 6 range, that is, the pressure difference between 1 X 10-6 and 1 X 10-5 Torr, involves a change in gas density of 10 times. We seem to think it is unimportant whether we are at 2 X 10-8 or 1 X 10-8 Torr: "Not to worry. It's okay. We're in the 8 range." Why does it matter if we pump to 2 X 10-8 rather than to 1 X 10-8 Torr? At the higher pressure, we will have twice the density of molecules present as at the lower pressure. If this presents no problem, why then are we spending time and money pumping to a lower pressure than required? If a pressure of 2 X 10-8 Torr is satisfactory, could we use 3 X 10-8 Torr and save some pumping time? After all, nature abhors a vacuum, and reducing the pressure by a factor of two or more does not come free." If the apparent sensitivity of an MS instrument is off 10–15%, or seems to vary erratically, a "small" change in pressure in the mass analyzer may be the cause. The author of this quote addresses the need for accurate pressure measurement in a vacuum chamber used for materials processing, but the lesson is valuable. For the mass spectrometer, scattering collisions reduce ion transmission, and reduce instrument sensitivity. A "small" change in pressure can lead to an amplified degradation in performance. Pumps "burp" for various reasons, and the short pressure surge takes time to dissipate. Erratic instrument performance may be your only clue if you are not carefully monitoring the pressure. The author may be the only mass spectrometrist in the world who would become concerned with a shift in measured base pressure from 2 X 10-6 to 3 X 10-6 Torr, but now you know why.
Performance of the vacuum pumping system of a mass spectrometer is monitored through measurement of the pressure at various points in the system. These measured pressures may or may not be logged into the automated control system of the mass spectrometer. In process vacuum chambers, system pressures certainly are recorded so that the conditions of materials processing are known precisely. But in analytical mass spectrometers, pressure measurement is often manually monitored. Consider Figure 1, which is a schematic of a diffusion pump attached to the main vacuum chamber, supported by the backing pump (also known as the rough pump), with the system exhaust routed to a hood. Pressures within the backing pump lines are monitored (using the thermocouple gauge) as an indicator of the total gas load on the system, and to ensure that the main diffusion pump is properly supported. The main chamber pressure is monitored with the ionization gauge. The diffusion pump can operate for a short time at higher backing pressures, but prolonged operation leads inevitably to a rise in main system pressure and a degradation of the pumping fluid. In a simple EI mass spectrometer, monitoring the two pressures (the backing pump line thermocouple gauge and the main chamber ionization gauge) usually is sufficient for reassurance that the instrument was properly pumped.
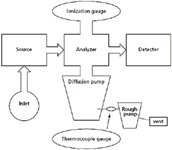
Figure 1: Simple diagram of placement of a thermocouple gauge (bottom) and an ionization gauge (top) on a generic mass spectrometer.
Details of how each measurement device works are explored in specialized texts (5–8) and commercial manufacturer's applications notes, reflecting the broad application of vacuum science outside of MS. We concentrate here on the thermocouple gauge and the ionization gauge, because these are the most common devices found on mass spectrometers, usually configured approximately as shown in Figure 1. Table I shows the operational pressure range for the thermocouple gauge and for the ionization gauge, and a few other devices included for comparison. Note that in addition to monitoring the pressure in the backing lines, thermocouple gauges also can be used to monitor vacuum pressures in sample introduction interfaces. Note that for sources that operate at atmospheric pressure, the ambient pressure usually is not measured. We emphasize in this column three basic issues relevant to the vacuum pumping system in a mass spectrometer. First, the vacuum gauge produces an electrical output that can be related to pressure through a calibration, and not all residual gas mixtures follow the same calibration curve. Second, the pressure measured is the pressure at the gauge, not necessarily in the system, and so we have to consider conductance. Third and finally, proper vacuum-pumping system care maintains instrument performance.
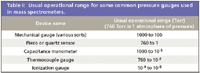
Table I: Usual operational range for some common pressure gauges used in mass spectrometers.
Calibration: Like any transducer, a vacuum gauge must be calibrated. In MS, that calibration entails some special issues. The composition of a gas mixture at Earth atmospheric pressure contains an expected mix of nitrogen, oxygen, water, carbon dioxide, argon, and some trace components. Does this mixture change in its relative composition as a pump lowers the pressure in a vacuum chamber? It most certainly does, because various pumps may act to pump one gas component more effectively than another. Thus the composition of that starting gas mixture at 10-3 Torr will be slightly different from the composition at atmosphere, even more different at 10-6 Torr, and vastly different at 10-9 Torr. For the pressure-measuring devices, a calibration should be completed so that the electrical output can be related to the actual pressure, and the calibration must factor in the composition of the gas. Any device reacts to different gases with a different sensitivity, and thus exhibits a different calibration curve. A thermocouple gauge that measures 1 Torr of pressure when the composition is residual atmospheric gases will be slightly different in response from a thermocouple gauge reading 1 Torr of methane in a CI source. Many commercial companies offer calibration services for vacuum gauges. In MS, traceability to a NIST or other national standard is not usually necessary. NIST calibration is described in detail in publications available on the web (9,10).
Conductance: The connection of a vacuum gauge to the mass spectrometer deserves some discussion. Consider the two situations depicted in Figure 1 for a thermocouple gauge and for an ionization gauge. The thermocouple gauge is connected directly into the vacuum line, and many gauges are built into threaded assemblies for such a purpose. We can be assured that the pressure measured by the thermocouple gauge is the pressure in the line because of this direct connection. On the other hand, consider the connection of an ionization gauge to the main vacuum chamber of a mass spectrometer. Sometimes the glass-enclosed ionization gauge is found in a T configuration equipped with a bolted vacuum machine flange. This vacuum flange is then bolted to the receiving flange teed off the main chamber. We want to know the pressure in the main chamber, but what we actually measure is the pressure at the gauge, which is related to the pressure in the chamber through the conductance of the connection. As a rule, instrument design should maximize conductance between the vacuum gauge and the chamber. For example, large diameter connections have better conductance than do smaller-diameter connections, and curved 90° bends have better conductance than do right-angle bends. To minimize possible issues of conductance, a direct mounting of the gauge is available with the use of a "nude" ion gauge. Here the analyst must be aware of the ionic and photonic emission of the gauge itself, as well as its fragility.
Care: A vacuum pumping system does not thrive on neglect, but this author's experience is that few currently practicing mass spectrometrists receive basic education in vacuum science. For example, a proper pumpdown technique (11,12) is part of the necessary care of a vacuum system and requires more attention than usually documented. Additionally, a good maintenance schedule includes, for example, attention to air filters, water filters, exhaust filters, flushing of water lines, pump oil replacement and proper disposal, seals testing, leak testing, replacements for degraded seals and hoses, and general system cleanliness. Pumps sited away from the mass spectrometer for purposes of noise abatement or vibration isolation are especially likely to fall off the maintenance schedule. "Bake-out" is a term that is not much used anymore, and it is doubtful that the reasons for completing one are still known. Finally, while source ionization cleanliness usually is acknowledged to be vital for good instrument performance, the cleanliness of the rest of the system (comprising the preponderance of surface area for the vacuum system) rarely is considered. Maintenance is not the most pleasant work of the MS laboratory, but it is one of the more important ones. Let the author know if you have assembled a workable reward structure that results in proper maintenance — "beer and pizza" will not be considered an adequate response.
Good vacuum system design is a crucial underpinning for high performance instrumentation. In "Mass Spectrometry Forum," our range of topics is extraordinarily broad, ranging from the basics of electronic and vacuum technology through to the ethical use of MS data. The important aspects of pressure and vacuum need regular teaching, and we will return to additional topics in these areas in future columns.
Kenneth L. Busch grew up in the era of belt-driven rough pumps. KLB always remembers to put a leak tray under his rough pumps, he knows what a sight glass is, and he knows the chemical molecular composition of many diffusion pump oils. In his youth, with a steadier hand, he could replace filaments in nude ionization gauges. Extra bonus points with no redeemable cash value are sent via email to those who deduce the pun not quite hidden in this column. No hints provided. Responsibility for this column resides solely with the author, who can be reached at: wyvernassoc@yahoo.com
References
(1) K.L. Busch, Spectroscopy 15(9), 22 (2000).
(2) K.L. Busch, Spectroscopy 16(5), 14, (2001).
(3) There are mass spectrometers that operate in the undersea environment, and also instruments that operate in the lower reaches of the atmosphere. Interfaces will differ, of course, but the basic pumping needs within the instrument itself remain similar. Outside that range, design considerations diverge.
(4) Granville-Phillips, Issues in Vacuum Measurement, "It's a Myth that Less Accuracy is Needed for Low-Pressure Measurement." Found at http://www.brooks.com/documents.cfm?documentID=4879.
(5) K. Jousten, Handbook of Vacuum Technology (Wiley-VCH, New York, New York, 2008).
(6) J.F. O'Hanlon, A User's Guide to Vacuum Technology (John Wiley and Sons, Hoboken, New Jersey, 2003).
(7) D.J. Hucknall and A. Morris, Vacuum Technology: Calculations in Chemistry, (Royal Society of Chemistry, UK, 2003).
(8) A very useful compendium of vacuum technology references is found at: http://www.atomwave.org/rmparticle/ao%20refs/aifm%20refs%20sorted%20by%20topic/beam%20detectors/Vacuum%20References.doc.
(9) S. Dittmann, "NIST Measurement Services: High Vacuum Standard and Use, 1989." Found at http://ts.nist.gov/MeasurementServices/Calibrations/upload/SP250-34.pdf
(10) J.H. Hendricks , P.J. Abbott, J.E. Ricker, J.H. Chow, and J.D. Kelley, "Development of a New NIST Calibration Service Using the Comparison Method for Vacuum Gauges Spanning the Range 0.65 Pa to 133 kPa," found at www.cstl.nist.gov/projects/fy06/indst0683608.pdf.
(11) See http://www.vacuumlab.com/Articles/VacLab32.pdf.
(12) See http://www.plasma.org/activity/groups/subject/vac/Events_Archive/file_8392.ppt.

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
AI-Driven Raman Spectroscopy Paves the Way for Precision Cancer Immunotherapy
April 15th 2025Researchers are using AI-enabled Raman spectroscopy to enhance the development, administration, and response prediction of cancer immunotherapies. This innovative, label-free method provides detailed insights into tumor-immune microenvironments, aiming to optimize personalized immunotherapy and other treatment strategies and improve patient outcomes.