Analysis of Deposition Patterns and Influencing Factors of Lithium and Transition Metals Deposited on Lithium Ion Battery Graphitic Anodes by LA-ICP-MS
A comprehensive understanding of aging phenomena and the resulting performance loss occurring in lithium-ion batteries (LIBs) is essential for ongoing improvement of the technology. The formation of a uniform solid electrolyte interphase (SEI) is of crucial importance for the performance, lifetime, and safety of LIBs. Transition metal dissolution (TMD), caused by degradation of the cathode, and subsequent TM deposition on the anode surface can deteriorate the protective properties of the SEI, possibly leading to reconstruction of the SEI and loss of active lithium.For layered cathode materials, different dissolution mechanisms have been proposed: impurities within the nickel-cobalt-manganese (NCM) host lattice (oxygen vacancy); particle cracking induced by structural deformation on the atomic scale; and phase transition from layered to spinel-type structures on the nanoscale level. Possible correlation of transition metal deposition patterns and increased lithium passivation or plating on the anode surface were visualized by elemental imaging using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS).
Despite the substantial investment into the lithium-ion battery (LIB) technology and its major advantages compared to other battery technologies and storage solutions, the constant demand for increasing performance needs to be met to fully realize the energy transition. Current research has focused on developing new materials, improving safety, and tailoring battery properties to meet the diverse and demanding requirements of applications like high-power tools and long-range electric vehicles (EVs), which are strongly influenced by cell chemistry and operating conditions. Crucially, the so-called “aging” of battery components and the resulting continuous performance loss represents a pivotal limitation of all lithium ion-based battery chemistries.
Consequently, a prominent strategy to increase LIB energy densities is to improve the performance of established materials by enhancing the materials electrochemical and thermal stability, allowing for operation at more demanding conditions, specifically increased cathode potentials and excess to higher practical capacities and cell voltages. The degradation of the electrodes, anode, and cathode respectively, are prominent examples of aging phenomena that are significantly accelerated by high voltage (HV) operation, frequently invoking drastic capacity fade and reduced battery life.
Therefore, a comprehensive understanding of the underlying aging processes occurring at the respective electrode-electrolyte interphases is essential to identify and develop suitable improvement strategies and enable safe and sustainable operation outside current electrochemical stability windows. Possible approaches might include solid electrolyte interphase (SEI)-modification via electrolyte additives, active material coatings to improve structural stability, or element doping of active materials. Sophisticated analytical post-mortem investigations allow insights into the complex aging processes and help to decipher underlying degradation mechanisms. More specifically, surface sensitive techniques, such as laser ablation-inductively coupled plasma–mass spectrometry (LA-ICP-MS) offer comprehensive imaging and depth-resolved analysis at different resolution scales. Spatial and depth-resolved elemental information allows for the study of different processes, such as the transition metal dissolution (TMD) and subsequent deposition on the anode surface, as well as the loss of active lithium (through deposition in the SEI lithium plating). Established techniques for these investigations include time of flight-secondary ion mass spectrometry (TOF-SIMS) and glow discharge–optical emission spectroscopy (GD-OES). However, small spot sizes, prolonged measurement times, or the inability to provide spatial resolved information, limit the applicability of these techniques. In comparison, LA-ICP-MS offers the unique combination of spatial and depth-resolved information, excellent limits of detection, fast measurement times (even for large samples), and easy sample preparation.
This work deals with the application of LA-ICP-MS as a direct solid sample analysis technique for the investigating the degradation of LIB electrode materials. Mapping experiments were carried out to visualize deposition patterns of lithium and transition metals, as well as study the factors influencing these depositions.
Materials and Methods
The pouch cells consisted of a cathode: NCM 111 (101 x 73 mm) and anode: graphite (104 x 76 mm) separated by a Celgard 2325 separator, which was wetted with 1 M LiPF6 in ethylene carbonate:ethyl methyl carbonate (EC:EMC) (3:7 by weight) and 2 wt% vinylene carbonate (VC) as the electrolyte. The cycling conditions can be found in Table I.
The cells were afterwards opened and disassembled in a glovebox. The anode was rinsed with dimethyl carbonate (DMC) to remove conducting salts residues from the electrolyte and subsequently dried before analysis. Afterwards, LA-ICP-MS investigations were carried out the analyze the transition metal and lithium distribution.
LA-ICP-MS
LA-ICP-MS investigations were utilized to visualize elemental distribution patterns across large sample areas (entire electrodes) after electrochemical aging. For LA-ICP-MS analysis, an Analyte Excite 193 nm ArF excimer laser system (Teledyne CETAC Technologies) equipped with a HelEx II ablation chamber (Teledyne CETAC Technologies) was employed. The instrument was hyphenated to a 7700x ICP-MS system (Agilent Technologies) via an Aerosol Rapid Introduction System (ARIS, Teledyne CETAC Technologies). The ARIS system consisted of specific transfer tubes to the ICP-MS and a solid-state nebulizer, limiting aerosol cloud expansion during shorter wash-out times. The respective instrument parameters are listed in Table II. Further information can be found via Schwieters and colleagues (1,2).
Results
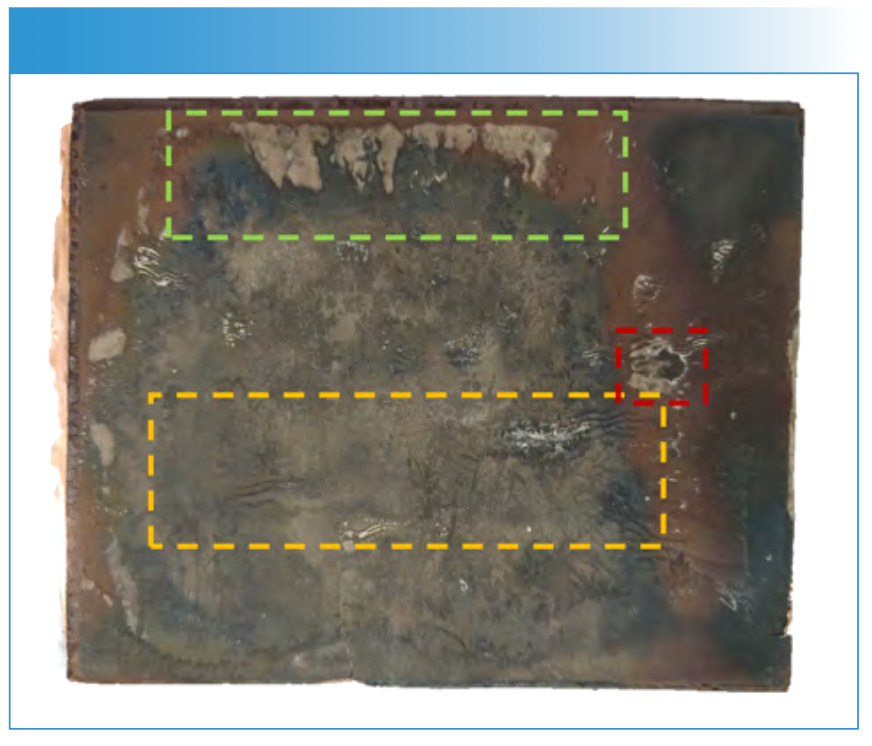
The LA-ICP-MS investigations were carried out in different areas of the anode. These areas already showed visible changes on the electrode surface (Figure 1). Overall, three areas (deposition area I–III) were investigated, and the deposition and distribution of lithium and the transition metals were analyzed.
FIGURE 1: View of the investigated anode. The different color region represent the areas, which were of interest for the LA-ICP-MS investigations (green = I; yellow = II and red = III).
Deposition Area I
The investigated area (green) was chosen because the surface of the anode already showed a visible deposition. These depositions can be assumed to be lithium plating (Figure 2); however, they needed to be verified by determining the lithium distribution.
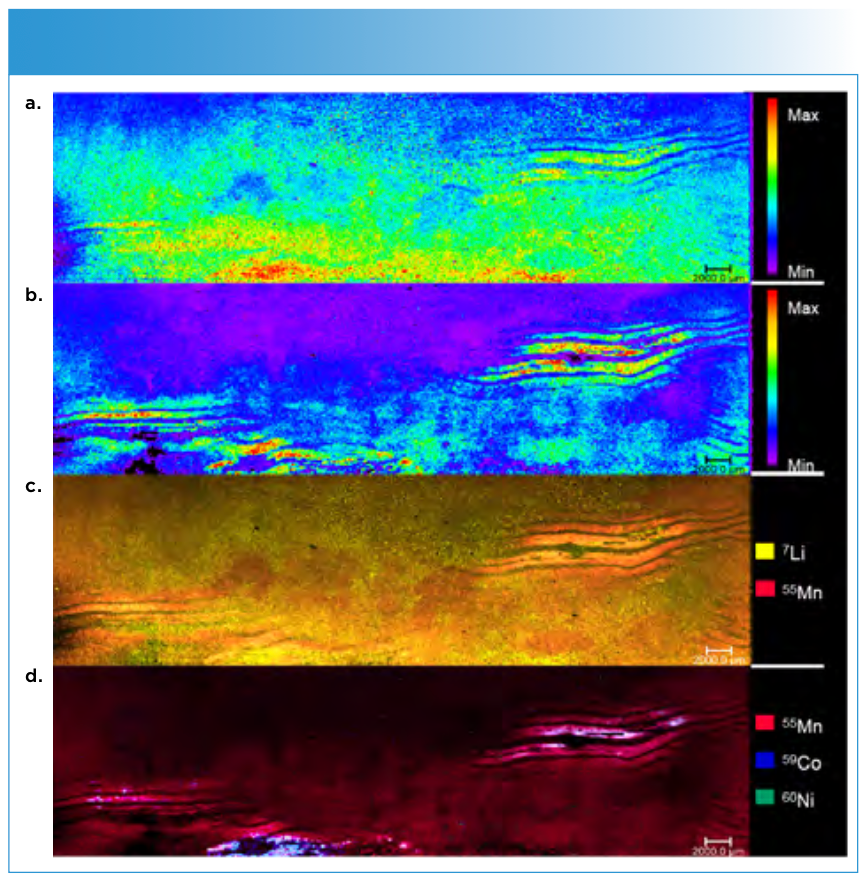
FIGURE 2: Mapping of the green deposition area I. The uppermost image (a) shows the overall signal intensities of the deposited elements. The next image (b) the overlay of 7Li and 55Mn, followed by the comparison of 7Li, 60Ni and 59Co (c). The last image shows the overlay of all three transition metals (d).
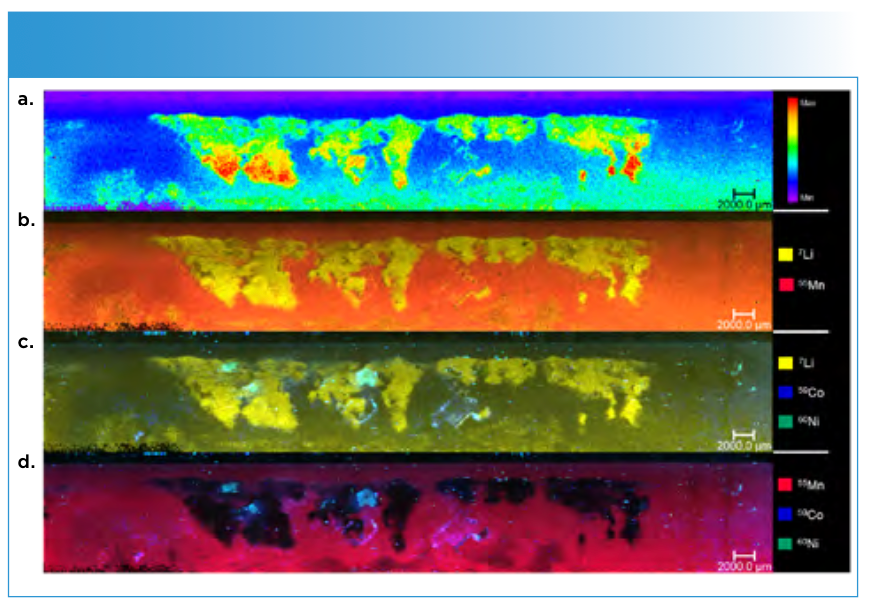
The corresponding LA-ICP-MS image (b) shows a distinct lithium pattern on the upper side of the electrode, matching the deposition visible on the electrode surface. Conducting salt residues can likely be excluded because of the washing step during the sample preparation.
Furthermore, it can be seen that the overlay shows an absence of manganese on the lithium deposition sides. In areas around the plating, the signals overlap. In comparison, the other two transition metals exhibit a different deposition behavior. Spots of nickel and cobalt are visible on some of the lithium plating areas (c). No obvious correlation between manganese, cobalt, and nickel is visible. Only partly matching depositions of cobalt and nickel can found, in contrast to normally assumed uniform depositions of the elements.
Deposition Area II
In the second area (Figure 3), wave-like deposition patters are visible throughout the area for all analytes of interest. In comparison to the previous are, the deposition of lithium, nickel, manganese and cobalt match very well. One reason for this behavior can be locally different pressure conditions, as this effect was already described in the literature. The effect seems to be less pronounced for lithium in comparison to the transition metals, matching previous results (2,3). Because of the pressure, the spatial distance between the electrodes is shorter, therefore resulting in enhanced deposition patterns. In this case, a rippled separator on the deposition sites can be responsible for inhomogeneous pressure distribution. The corrugated separator could be originating from the assembling process or possibly from local gas emissions. The separator was later identified as the source of the wave-like pattern because of additional experiments with a rippled separator during the assembling process and further post mortem characterizations.
FIGURE 3: Image of the wave-liked deposition patterns. Different overly between lithium and the transitions metal are presented. Figures 3a and 3b show the overall signal intensities of the deposited elements, while (c) depicts the overlay of 7Li and 55Mn and (d) the distribution of all three transition metals.
Deposition Area III
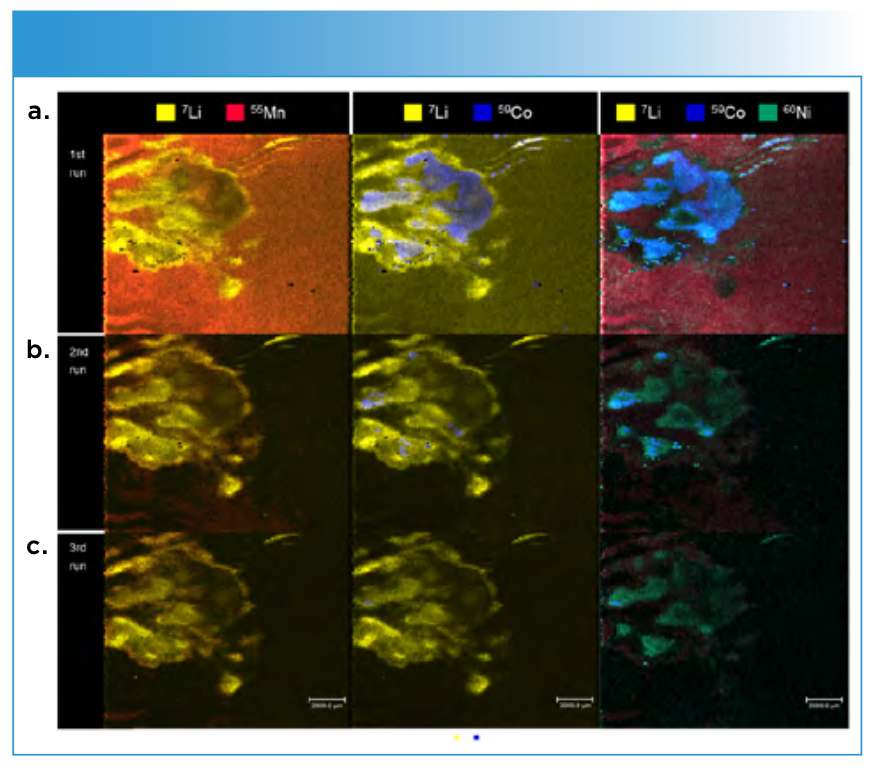
Area III was chosen to analyze the deposition pattern in the depth for the different elements. Because two different deposition behaviors were found in area I and II (Figure 4), a region with no wave-like pattern was chosen. By comparing the images of subsequent ablation runs (depth profile), a decrease in signal intensities for all analytes can be observed. However, this effect is less distinct for lithium. No matching deposition of lithium or any of the transition metals can be observed. Cobalt notably deposits in “lithium gaps” with distinct boundaries. An overlapping of cobalt and nickel is observable, whereas the manganese signal does not correlate with the other transition metals (TMs).
FIGURE 4: Images of the different ablation depths for lithium, nickel, cobalt, and manganese. 1st run (a), 2nd run (b) and 3rd run (c).
Conclusion
In general, it could be shown that area II showed matching deposition of Li and all TMs, while the effect was more severe for TMs. This was caused by inhomogeneous pressure distribution, resulting in higher signals in high pressure regions. Here, a rippled separator was the source of the pressure regions, showing potential for the method to investigate production problems.
In comparison, no correlation between Li and TM (especially Mn) could be observed. The lithium plating might prevent the deposition of transition metals. This trend is consistent for deeper layers, as observed in the depth profile.
The presented work illustrates the promising potential of the LA-ICP-MS method for lithium ion battery electrodes. It allows fast and accurate imaging of main. The capability of the method for depth and lateral distribution investigations opens broad perspectives for future investigations. Despite the complex analysis of layered structures of electrodes with surface films, the method was applied successfully to study the depth profile of TM species in/at/on graphite electrodes. Further investigation into lithium plating and the possible prevention of TM deposition using higher resolved depth resolution techniques (for example, TOF-SIMS) is needed. The imaging of separators on both types of deposition sites may identify TM migration pathways through the electrolyte and thus provide possible reasons for the deposition behavior.
Acknowledgement
The authors would like to thank the Ministry of Economic Affairs, Innovation, Digitalisation and Energy of the state North-Rhine-Westphalia for funding the “GrEEn” project (313-W044A).
References
- Schwieters, T.; Evertz, M.; Mense, M.; et al. Lithium Loss in the Solid Electrolyte Interphase: Lithium Quantification of Aged Lithium Ion Battery Graphite Electrodes by Means of Laser Ablation Inductively Coupled Plasma Mass Spectrometry and Inductively Coupled Plasma Optical Emission Spectroscopy. J. Power Sources 2017, 356, 47–55. DOI: 10.1016/j.jpowsour.2017.04.078.
- Schwieters, T.; Evertz, M.; Fengler, A.; et al. Visualizing Elemental Deposition Patterns on Carbonaceous Anodes from Lithium Ion Batteries: A Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry Study on Factors Influencing the Deposition of Lithium, Nickel, Manganese and Cobalt after Dissolution and Migration from the Li1[Ni1/3MN1/3CO1/3]O2 and Limn1.5 Ni0.5O4 Cathode. J. Power Sources 2018, 380, 194–201. DOI: 10.1016/j.jpowsour.2018.01.088.
- Harte, P.; Evertz, M.; Schwieters, T.; et al. Adaptation and Improvement of an Elemental Mapping Method for Lithium Ion Battery Electrodes and Separators by Means of Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry. Anal. and Bioanal. Chem. 2018, 411 (3), 581–589. DOI: 10.1007/s00216-018-1351-9.
Patrick Harte, Martin Winter, Simon Wiemers-Meyer, and Sascha Nowak are with the MEET Battery Research Center at the University of Münster in Münster, Germany. Martin Winter is also with the Helmholtz-Institute Münster, in Münster, Germany. Direct correspondence to: sascha.nowak@uni-muenster.de ●

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.
Best of the Week: Hyperspectral Imaging, ICP-MS Analysis of Geological Samples, Product Roundup
October 18th 2024Top articles published this week include an article about hyperspectral imaging in human skin research, a peer-reviewed article about analyzing geological samples using atomic spectroscopy techniques, and an equipment roundup piece about the latest products in the industry.