Application of Raman Spectroscopy to Lubricants, Lubricated Surfaces, and Lubrication Phenomena
Developments in lasers, detectors, low-cost instruments, and fiber-optic probes have greatly expanded the lubrication systems being studied by Raman spectroscopy.
Recent advances in lasers and detector technologies and the development of low-cost instruments and fiber-optic probes have brought about an explosion in the lubrication systems being studied by Raman spectroscopy. This article provides examples of the use of Raman spectroscopy in the study of lubrication phenomena, including the characterization of liquid lubricants, lubricant additives, and solid lubricants; the study of vapor-phase lubrication; and the use of coated nanoparticles as lubricants.
Raman spectroscopy has long been an important tool for the analysis of lubricants (1) and lubricant additives (2). The technique is well suited for use in lubrication because the highly focused incident beam allows for the study of phenomena in constrained spaces. Also, the fact that Raman cells can be constructed from durable materials such as glass adds to Raman's applicability to the study of lubricants, in contrast to infrared spectroscopy where cells are typically made of salts such as sodium chloride. In addition, many of the materials studied in the lubrication field are solids or coatings on solids that can be difficult to analyze by other spectroscopic techniques, especially infrared spectroscopy. Raman spectra have been shown to contain a wealth of information about the details of interactions among lubricants, lubricant additives, and bearing materials.
Recent developments in Raman instrumentation have greatly expanded the range of lubrication systems that can be studied. The development of inexpensive detector arrays, for example, has decreased the cost of Raman instrumentation while increasing the capabilities of the instrumentation to detect small signals. Improved coupling of Raman instrumentation with microscopy, furthermore, has made it easier to study phenomena occurring in small areas of the sample. The development of stable diode lasers also has expanded the types of systems that can be studied by reducing fluorescence interference through the use of a red or infrared laser. Finally, the ability to couple Raman instruments to fiber-optic probes allows for the study of phenomena that occur under extreme conditions of temperature and pressure (3). These advances have brought about an explosion in Raman's use to study lubrication phenomena.
This article provides examples of the use of Raman spectroscopy in the study of lubrication phenomena, including the use of Raman to characterize liquid lubricants, lubricant additives, and solid lubricants, and to study vapor-phase lubrication and coated nanoparticles as lubricants. It is not intended to be an exhaustive review of all of the literature.
Liquid Lubricants and Lubricant Additives
Liquid lubricants typically consist of a liquid base stock combined with a package of additives that contains antioxidants, metal deactivators, viscosity modifiers, and extreme pressure or antiwear additives. One study used changes in the Raman spectrum of the lubricant as a probe of the pressure on entrained lubricant in the bearing entrapment region. The changes in Raman shift indicate pressures of up to 12 kbar (4). Another study demonstrated the extreme conditions that the base stock of liquid lubricants must withstand (5).
Numerous studies of additives also have made use of Raman spectroscopy in various ways. The structures of some common additives are shown in Figure 1. Several studies have used surface-enhanced Raman spectroscopy to study lubricants and additives at copper surfaces. In particular, stearic acid was found to bind to a copper surface in a perpendicular manner, before being converted to copper(II) stearate (6). Raman studies of 1,3,4-thiadiazole bis disulfide–containing additives that act as copper deactivators demonstrated that the active sulfur is converted to trisulfides at the surface (7). Raman spectroscopy further demonstrated that the ringed structure remained.
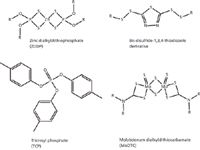
Figure 1: The structure of several common lubricant additives.
Many extreme-pressure additives have been examined using Raman spectroscopy. These additives typically react at bearing surfaces to form a durable, lubricious coating. Surface coatings are often characterized by Raman spectroscopy, because the soluble additive is frequently not the same as the deposited coating; for example, dimethyl disulfide is converted to iron(II) sulfide at the surface (8).
Zinc dialkyl dithiophosphate is a common extreme-pressure additive used in automotive applications. In one study, Raman spectroscopy was combined with internal reflectance infrared spectroscopy to examine the effect of aging on additives in motor oil. The surface films formed using fresh oil contained calcium carbonate, calcium phosphate, and sulfides. Upon aging, the film changed to primarily zinc and iron phosphates and elemental carbon. The study also demonstrated that infrared and Raman spectroscopy provide complementary information, with infrared being more sensitive to ionic vibrations and Raman more sensitive to the molecular vibrations (9). In another study, Raman spectroscopy was used to identify MoS2 in the surface film from the additive molybdenum dialkyldithiocarbamate (10).
Phosphate esters are another class of extreme-pressure or antiwear additives that have found broad application. Raman spectroscopy is one of many techniques that have been used to identify surface species. The Raman spectrum of tricresyl phosphate (TCP), iron(II) phosphate, and the film deposited on a metal coupon are shown in Figure 2. The spectra show that the film contains phosphate peaks in the region around 1000 cm-1 . The peaks in the metal coupon are shifted to lower energy, indicative of the formation of a polyphosphate. Similar films formed from the deposition of zinc orthophosphate show a similar broadening and shifts in energy (11). The peaks are broadened, suggestive of multiple forms of phosphate such as polyphosphates. The spectra also show a broad peak at about 1600 cm-1 , which is characteristic of sp2 -hybridized carbon. Surface analysis of the films is consistent with the formation of low-order graphite as a layer between the phosphate film and the bulk of the metal. The surface films formed are discussed further in the section on vapor-phase lubrication.

Figure 2: Raman spectra of iron(II) phosphate and tricresyl phosphate reference samples (top) and the film formed on two metal coupons (bottom).
Bearing Materials
The desire to improve engine performance has led to the development of new materials that can withstand higher temperatures and higher loads. Materials that have been developed include diamond-like carbon (DLC) coatings, carburized steels, carbide or nitride coatings, and ceramic bearings. Raman spectroscopy has played an important role in examining the interactions of lubricants with these new materials.
A number of new metal alloys under development for bearing applications are carburized steels. These materials are characterized by a surface that contains a high concentration of metal carbides. The interaction of phosphate esters, as a typical extreme-pressure additive with carbide surfaces, has been shown by Raman spectroscopy to form surface polyphosphate coatings. The broad peaks observed indicate a significant amount of randomness in the arrangement of the polyphosphates and the degree of polymerization (14).
When metal carbides are codeposited with amorphous carbon and the interfaces are studied by confocal Raman spectroscopy, metal oxides are identified in the Raman spectra for samples with low mole fractions of amorphous carbon on the surface. If higher mole fractions of amorphous carbon are present, Raman peaks characteristic of diamond (1375 cm-1 ) and graphite (1580 cm-1 ) are observed. The study concluded that sufficient amorphous carbon must be present to form a lubricant amorphous carbon phase (15).
Titanium nitride coatings have also been studied using near-infrared Raman imaging. This study indicates that there is significant titanium oxide formed under moderate loads (16). In the study of titanium aluminum oxide, however, Raman was not able to identify a thin layer of aluminum oxide on the surface that was observed by other techniques (17).
Diamond-like carbon is a particularly promising coating for use as a bearing material. As the name implies, the hardness of the coating should resemble that of a diamond. However, Raman spectroscopy indicated a significant amount of sp2 -hybridized carbon at the surface, consistent with at least a thin coating that is more graphitic in nature (18). In several studies, Raman spectroscopy has indicated that diamond-like coatings of various sources have substantial distortion of the diamond-like structure. This is indicated by shifts in energy and broadening of the peak at 1332 cm-1 , which indicates sp3 -hybridized carbon, and the presence of large broad peaks at 1580 cm-1 indicative of sp2 -hybridized carbon. The sp2 -hybridized carbon would be considered graphitic, and possibly disordered (19). Raman studies also have demonstrated that in unlubricated conditions, a graphitic layer is present to substantially reduce friction (20). In addition, details of the preparation and the seeding of the film were shown to influence the distribution of sp2 and sp3 carbon through changes in Raman intensities and energies (21).
Solid Lubricants
Solid lubricant coatings applied in numerous ways to solid substrates have many desirable properties. Raman spectroscopy has been used extensively to study solid lubricants and their reactions under wear conditions (22). A wide range of oxides and sulfides has been studied by Raman spectroscopy. A particularly interesting solid lubricant studied by Raman spectroscopy is cesium oxythiomolybdate. Raman spectra collected as a function of temperature indicated that the material at 25 °C was not a pure compound, but a mixture that also contained Cs2SO4, Cs2MoO4, MoO3, and MoS2. Raman spectra collected as a function of temperature showed dramatic changes in composition, with the material completely transformed to cesium oxides and molybdenum oxides at 800 °C (23). The situation increased in complexity when the lubricant was used with a silicon nitride substrate. In addition to demonstrating that there were changes in the lubricant (seen in changes in the Raman spectrum at high temperature), Raman spectroscopy also indicated changes in the composition of the substrate, converting silicon nitride to silicon oxide and other materials (24).
Recent studies have focused on the changes that occur in lubricants during high-temperature operation. In a study of molybdenum sulfide at high temperatures, molybdenum oxide was detected just before the failure of the lubricant (25). The formation of a small amount of molybdenum oxide resulted in a large increase in the friction in the bearing. In the same study, a vanadium nitride silver nanocomposite coating was observed by Raman spectroscopy to react to form silver vanadate phases (Ag3VO4 and AgVO3). The formation of the silver vanadate phases resulted in a much more gradual increase in friction. Real-time Raman spectroscopy indicated that the composition of the coating changed upon heating to
1000 °C to a mixture of vanadium oxide, metallic silver, and silver vanadate. Upon cooling to 400 °C, the silver and vanadium oxide had reverted to silver vanadate (26).
Vapor-Phase Lubrication
Vapor-phase lubrication relies on the delivery of the lubricant as a vapor or mist to the bearing surface. At high temperatures, the lubricant reacts with the surface to form a solid coating that acts as a lubricant and is worn away. The coating is continuously replaced by a continuous supply of the lubricant. A number of different systems have been studied as vapor-phase lubricants, including carbon-containing gases and phosphate esters.
Carbon-containing gases such as ethane, ethylene, acetylene, benzene, and carbon monoxide have potential use as carbon-containing vapor-phase lubricants at high temperatures. Raman investigations have focused on the nature of the deposits and on the effect of the high temperatures and pressures generated in the physical contact zone. Raman spectroscopy has shown that for all of these gases, the lubricant outside and within the wear track has vibrational energies similar to those of graphite (27). The similarity of the Raman spectra recorded inside the wear track and outside the wear track indicates that the sliding motion of the pin does not alter the chemical structure of the carbon layer. Comparable studies of ceramic bearings show differences in the Raman spectra depending upon the temperature and feed gases used (28).
Vapor-phase lubrication of various types of steel surfaces has been shown to be a complex process that depends on the degree of oxidation of the surface (29). Raman spectroscopy has shown that under high-temperature and low-oxygen conditions, a layer of low-order graphite is formed between the metal surface and a phosphate-containing layer (30). Under more oxidizing conditions, Raman and surface spectroscopy indicate the presence of a polyphosphate layer with pendant aromatic rings on the surface. Raman spectroscopy is uniquely suited to the analysis of these surfaces because of its ability to observe highly symmetric vibrational modes, rather than the asymmetric modes observed by infrared spectroscopy.
Nanoparticles and Coated Nanoparticles
Since the discovery of fullerenes, there has been an interest in using nanoparticles in lubrication systems, either as a coating or as a lubricant additive. Much of the work has been focused on either carbon nanotubes or carbon-coated metal particles. Raman spectroscopy has been used as a probe of the structure of the carbon layer, and also as a probe of the materials present in the wear scar.
The completely carbon-based systems under recent study include graphene, graphene oxide, and carbon nanotubes in lubrication. One study of nanotubes as an additive to an ionic liquid used Raman spectroscopy to show the nanotube structure during wear (31). In particular, the authors examined the ratio of graphite (sp2 ) to diamond (sp3 ) carbon in the nanotubes (32). A separate study, in which graphene oxide and reduced graphene oxide were bound to a silicon substrate, used Raman spectroscopy to show that the two coatings had similar but slightly different diamond:graphite ratios (33). In a study of a graphite flake–polyimide composite system, Raman spectroscopy was used to study changes in the polyimide upon wear. The polyimide was partially converted to free carboxylic acids as the bearings were worn at high temperatures (34). In efforts to avoid the aggregation commonly found with fullerenes and carbon nanotubes, carbon nanopearls with a multilayer carbon shell structure were prepared by nickel catalyzed chemical vapor deposition. Raman spectroscopy showed the presence of both sp2 and sp3 carbon in the nanoparticles. An increase in the sp2 carbon stretching frequency was also observed (35).
Raman spectroscopy was also used to study some composite nanoparticle systems. A carbon nanotube–reinforced nickel matrix composite was laser engineered into a monolithic shape. After wear tests, Raman spectroscopy was used to examine the composite system and a system that did not include the nanotubes. In the absence of the nanotubes, the wear scar showed wear in the substrate as indicated by iron oxides or nickel oxide at the surface. In the test including nanotubes, the surface was made exclusively of carbon-based materials (36). In a related study, a carbon shell over a nickel core was studied as a solid lubricant. Raman spectroscopy was used to confirm that the carbon coating contained a mixture of graphitic and diamond forms of carbon (37).
Conclusion
The studies reviewed here indicate some of the varied uses of Raman spectroscopy in the study of lubrication phenomena. In each of these studies, Raman provides key information about chemical structures not available from other techniques. These results arise in part from the advantages Raman enjoys over infrared spectroscopy in sensitivity and sampling, and in part from the amount of molecular information that can be discerned from a Raman spectrum. Other techniques can provide images of surfaces and the elemental composition of a surface, but do not allow the analysis of chemical species at the surface. Given the importance of molecular structures in lubrication, Raman spectroscopy will continue to be used extensively to study lubricants and lubricated surfaces in the future.
References
(1) M. Ahmadjian and C.W. Brown. Anal. Chem. 48, 1257–1259 (1976).
(2) G.E. McManis and L.E. Gast, J. Am. Oil Chemists Soc. 51, 198–199 (1974).
(3) I.R. Lewis and M.L. Lewis, in Handbook of Vibrational Spectroscopy (John Wiley and Sons, New York, 2006).
(4) D.J. Gardiner, E. Baird, A.C. Gorvin, W.E. Marshall, and M.P. Dare-Edwards, Wear 91, 111–114 (1983).
(5) D.J. Gardiner, E.M. Baird, and C. Craggs, Lubrication Science 1, 301–313 (1989).
(6) Z.S. Hu, S.M. Hsu, and P.S. Wang, Tribol. Trans. 35, 417–422 (1992).
(7) Y.H. Luo, B.Y. Zhong, and J.C. Zhang, Lubrication Eng. 51, 293–296 (1995).
(8) J. Lara, T. Blunt, P. Kotvis, A. Riga, and W.T. Tysoe, J. Phys. Chem. B 102, 1703–1709 (1998).
(9) D. Uy, S.J. Simko, R. Carter, R.K. Jensen, and A.K. Gangopadhyay, Wear 263, 1165–1174 (2007).
(10) J. Graham, H. Spikes, and S. Korcek, Trib.Trans. 44, 628–636 (2001).
(11) W.D. Phillips, D.C. Placek, M.P. Marino, "Neutral Phosphate Esters," in Chemical Industries, 111 (Synthetics, Mineral Oils, and Bio-Based Lubricants), H. Heinemann, Ed. (CRC Press, Boca Raton, Florida, 2002), pp. 75–103.
(12) M. Gauvin et al., Tribol. Lett. 31, 139–148 (2008).
(13) D.W. Johnson, S. Morrow, N.H. Forster, and C.S. Saba, Chem. Mater. 14, 3867–3875 (2002).
(14) D.W. Johnson, J.E. Hils, and N. Forster, Trib. Lett. (2011), DOI 10.1007/s11249-011-9766-x.
(15) J.C. Sanchez-Lopez, D. Martinez-Martinez, M.D. Abad, and A. Fernandez, Surf. Coat. Technol. 204, 947–954 (2009).
(16) M. Lindquist, O. Wilhelmsson, U. Jansson, and U. Wiklund, Wear 266, 988–994 (2009).
(17) K.N. Jallad, and D. Ben-Amotz, Wear 252(11–12), 956–969 (2002).
(18) V. Marinez-Noques, F.J. Medel, M.D. Mariscal, J.L. Endrino, J. Krakowski, F. Yubero, and J.A. Puertolas, J Phys., Conference Series, 252 (2010), doi:10.1088/1742- 6596/252/1/012006.
(19) D. Zhang, B. Shen, and F. Sun, Appl. Surf. Sci. 256, 2479–2489 (2010).
(20) O. Jarry, C. Jaoul, P. Tristant, T. Merle-Méjean, M. Colas, C. Dublanche-Tixier, H. Ageorges, C. Lory, and J.-M. Jacquet, Plasma Processes Polym. 6(S1), S478–S482 (2009).
(21) V. Baranauskas, A.C. Peterlevitz, H.J. Ceragioli, A.L. Souto, and S.F. Durrant, Thin Solid Films 398–399, 255–259 (2001).
(22) J.L. Lauer, "Solid lubrication studied by optical means" Report, ARO-23760-2 (1989).
(23) K.L. Strong, J.S. Zabinski, and A.J. Vreugdenhil, J. Mat. Sci. 36, 5407–5413 (2001).
(24) K.L. Strong and J.S. Zabinski, J. Mat. Sci. 36, 5415–5422 (2001).
(25) C. Muratore, J. Bultman, S. Aouadi, and A. Voevodin, Wear 270(3–4), 140–145 (2011).
(26) S.M. Aouadi, D.P. Singh, D.S. Dtone, K. Polychronopoulou, F. Nahif, C. Rebholz, C. Muratone, and A.A. Voevodin, Acta Mater. 58, 5326–5331 (2010).
(27) N. Argibay, J. H. Keith, B.A. Krick, D.W. Hahn, G.R. Bourne, and W.G. Sawyer, Trib. Lett. 40(1), 3–9 (2010).
(28) J.L. Lauer and S.R. Dwyer, Trib. Trans. 34, 521–528 (1991).
(29) C.S. Saba and N.H. Forster, Trib. Lett. 12, 135–146 (2002).
(30) N.H. Forster, Tribol. Trans. 42, 1–9 (1999).
(31) G.M. Gong, Z. Li, Z. Zhang, B. Wu, X. Zhou, Q.Z. Huang, and J. Liang, Tribol. Int. 39, 937–944 (2006).
(32) F.J. Carrion, J. Sanes, M.D. Bermudez, and A. Arribas, Tribol. Lett. 41, 199–207 (2011).
(33) J. Ou, J. Wang, S. Liu, B. Mu, J. Ren, H. Wang, and S. Yang, Langmuir 26, 15830–15836 (2010).
(34) P. Samyn and G. Schoukens, Carbon 46, 1072–1084 (2008).
(35) C.N. Hunter, M.H. Check, C.H. Hager, and A.A. Voedvodin, Tribol. Lett. 30, 169–176 (2008).
(36) T.W. Scharf, A. Neira, J.Y. Hwang, J. Tiley, and R. Banerjee, J. Applied Physics 106, 013508-1–103508-7 (2011).
(37) V. Sunny, D.S. Kumar, Y. Yoshida, M. Makarewicz, W. Tabis, and M.R. Anantharaman, Carbon 48, 1643–1651 (2010).
David W. Johnson is a professor of chemistry at the University of Dayton, in Dayton, Ohio. He can be contacted at dave.johnson@notes.udayton.edu

AI-Powered SERS Spectroscopy Breakthrough Boosts Safety of Medicinal Food Products
April 16th 2025A new deep learning-enhanced spectroscopic platform—SERSome—developed by researchers in China and Finland, identifies medicinal and edible homologs (MEHs) with 98% accuracy. This innovation could revolutionize safety and quality control in the growing MEH market.
New Raman Spectroscopy Method Enhances Real-Time Monitoring Across Fermentation Processes
April 15th 2025Researchers at Delft University of Technology have developed a novel method using single compound spectra to enhance the transferability and accuracy of Raman spectroscopy models for real-time fermentation monitoring.
Nanometer-Scale Studies Using Tip Enhanced Raman Spectroscopy
February 8th 2013Volker Deckert, the winner of the 2013 Charles Mann Award, is advancing the use of tip enhanced Raman spectroscopy (TERS) to push the lateral resolution of vibrational spectroscopy well below the Abbe limit, to achieve single-molecule sensitivity. Because the tip can be moved with sub-nanometer precision, structural information with unmatched spatial resolution can be achieved without the need of specific labels.