Combating Terrorism with Mass Spectrometry — Screening People for Explosives
In this article, the authors review the capabilities and challenges of deploying mass spectrometry (MS) to homeland security screening requirements.
In this article, we review the capabilities and challenges of deploying mass spectrometry (MS) to homeland security screening requirements. The fidelity of MS for chemical analysis is well accepted; however, the effectiveness of an overall system in real-world work environments depends on solving big challenges in sample collection, speed of analysis, and ease-of-use, and maintaining continuous reliability. We compare MS to the more commonly used security method of ion mobility spectrometry (IMS) and illustrate the significant improvements that MS can provide. We then describe one of the first introductions of MS in homeland security, that being the screening of people for concealed explosives using a walk-through portal device.
The high flow of traffic in airports and mass transportation venues presents an enormous challenge for screening the premises for terrorist devices. Furthermore, as terrorists become more sophisticated, there is a need to detect an increasing variety of threats (such as improvised and homemade explosives, and chemical weapons). A broad range of countermeasures are needed, one of which is detection technology to screen against concealed explosives. In this article, we review the topic of explosives trace detection (ETD) and describe the method of mass spectrometry (MS) as an alternative to existing technologies.
There are two basic types of explosives screening systems. "Bulk" detection involves looking inside of objects using methods like transmission and backscatter X-ray and X-ray tomography, but also includes millimeter wave backscatter and nuclear quadrupole resonance. Bulk detectors generally look for concealed objects that appear suspiciously like explosives (that is, anomalies) but do not make a positive identification for explosives. "Trace" detection includes methods of chemical analysis that can make positive identification of explosives, but they cannot probe inside objects very easily. Instead, they search for traces of contamination on objects that might be carrying explosives. The most commonly deployed trace detectors in security today are based on ion mobility spectrometry (IMS), which we will compare to MS shortly.
One of the greatest vulnerabilities in aviation and other security venues is detection of concealed explosives on people. Whereas all baggage that goes on a plane (checked and carry-on) gets X-rayed at a minimum, people are generally only checked for possession of metal objects. Over the last several years, a number of trace detection systems for people, or portals, were developed and deployed. The first of these, called "puffer" machines, use IMS. Their performance was limited to just a few explosives compounds. The Transportation Security Administration (TSA) and the Department of Homeland Security (DHS), seeking greater trace detection capability, have supported the development of an MS-based portal device. In this article we will review the progress made in deploying MS for homeland security needs with an emphasis on the portal developments. Although commercial MS systems have been deployed for other threat situations, such as detecting chemical weapons in battlefields (1) or in water supplies (2), the Guardian portal (Syagen Technology, Tustin, California) is the first commercial MS system deployed for security screening of explosives (3). We will also describe other MS developments that could lead to deployed systems and note the long-term importance of MS in detecting explosives for other applications such as forensics (4).
Explosives Trace Detection Methods
Chemical Analyzers
There are many different analytical techniques available for the detection of explosives (5) because no single detector is suitable for all applications. The level of accuracy is somewhat a function of size, weight, and power requirements. Fixed location applications can use more sophisticated technology, whereas portability imposes compromises in performance.
MS offers high levels of sensitivity and specificity compared to other technologies for chemical detection. Its traditional disadvantages have been high cost and complexity. Over the last few years, however, the economics and robustness of key MS components have greatly improved and MS is now capable of routine and automated operation.
IMS is similar in concept to MS except that the ions are dispersed by gas-phase viscosity and not by molecular weight. The main advantage of IMS is that it does not use a vacuum system, which greatly reduces the size, cost, and complexity relative to MS. However, the trade-off is that the measurement accuracy is considerably less than MS. This is especially true for complex samples or when screening for a large number of target compounds simultaneously. New developments, such as field asymmetric ion mobility spectrometry (FAIMS), are improving on detection accuracy of IMS (6). Other methods, such as gas chromatography (GC), infrared (IR), Raman, and chemical sensors, have also been deployed; however, we will limit our discussions to MS and its variant, IMS.
The explosives that are of greatest concern for screening purposes are the high explosives (for example trinitrotoluene [TNT], cyclotrimethylene trinitramine [RDX], pentaerythritol tetranitrate [PETN]) and their composition forms (such as C-4, Semtex H, Detasheet), dynamites (such as nitroglycerin [NG] and ethylene glycal dinitrate [EGDN]), improvised explosives (such asammonium nitrate [AN], ammonium nitrate fuel oil [ANFO], urea nitrate, triacetone triperoxide [TATP], and black and smokeless powder), and ICAO taggant compounds (such as 2,3-dimethyl 2,3-dinitrobutane [DMNB] and EGDN). However. a host of other explosives threats are in the hands of terrorists (such as cyclotetramethylene tetranitramine [HMX], hexamethylene triperoxidediame [HMTD], black powder derivatives, and urea nitrate [UN]). In fact, the U.S. Bureau of Alcohol, Tobacco, and Firearms lists over 200 explosives materials (7).
MS as a Screening Tool
MS is an important analytical tool for quantitative analysis, where accuracy is defined by the capability to measure a concentration or mass quantity of a known compound in some sample, such as a solution. Because the unknown concentration can conceivably span a large concentration range, analyzers with large dynamic range are essential for quantitative analysis.
Screening analysis does not require determining precise concentrations, but instead must give a reliable indication of whether a target compound is present above some threshold concentration. The accuracy of a screening system is based on the probability of detection (PD) of the target compound at the threshold concentration relative to the probability of false positive (PFP) due to background compounds giving a signal that appears indistinguishable to the target signal. Although screening does not require as high a dynamic range as that needed for quantitative analysis, it is still an advantage because it can minimize the effect that a strong background signal can have on weak target signals and it can help resolve between strong background and strong target signals that might overlap.
Personnel Screening
The Guardian explosives trace portal (ETP) is based upon MS detection. Trace portals are also supplied by GE Security (Bradenton, Florida) and Smiths Detection (Danbury, Connecticut) and are based upon IMS detection. The method of sampling individuals for all trace portals is essentially "sniffing" their exterior (their clothing and skin) for contamination from handling explosives. Because the vapor pressures of explosives are very low, the sample that is collected consists of particles and residue. Sampling is done by impinging the individual with air jets to shake loose the particles and then flowing the large air volume surrounding the person through a concentrator to catch the loosened particles (8). The concentrator used in the Guardian portal consists of a metal mesh and is based on a design developed by Sandia National Laboratories (Albuquerque, New Mexico). The sample collected on the metal mesh is then thermally desorbed by passing a current through the mesh. The vaporized sample is then analyzed by the MS analyzer.
Unfortunately, there are not many publicly released test results for portals (test results are generally classified). Testing typically is done by two methods. TSA testing is conducted using patches of cloth upon which calibrated quantities of explosives are deposited. These patches are adhered to various locations on people to test for collection and detection efficiency. The quantities of explosives placed on these patches represent levels of contamination the TSA has determined are typical for bomb carriers. Another method primarily used by overseas users is to place bulk explosives on individuals and test for the detection of actual contamination.
Mass Spectrometry System
The ETP MS system discussed previously is based upon quadrupole ion trap, time-of-flight (QitTOF) MS and was first developed by Lubman and coworkers (9,10) and commercialized by Syagen (2,11,12) and Shimadzu (Kyoto, Japan) (13). A schematic of the QitTOF configuration is shown in Figure 1. The principal role of the ion trap is to accumulate ions that are continuously generated by the ionizer. These accumulated ions are then pulsed into the TOFMS analyzer at relatively high repetition rates (10–60 Hz). Because the TOFMS analysis is performed independently of the ion accumulation into the trap other than the short RF shutoff and pulse out time (about 50–100 μs), the duty cycle for collecting ions in the total MS analysis cycle is >99%. This is to be compared with an IT MS analyzer, where duty cycle is typically <50% because the accumulated ions must be scanned out of the ion trap, which typically takes on the order of 100 ms.
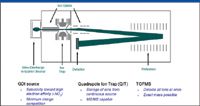
Figure 1: Schematic of the QitTOF MS detection system showing the individual components and their respective advantages.
The main benefits of QitTOF relative to other MS systems are
- TOFMS analyzes all ions at once versus quadrupole MS, which is a scanning device that detects only one ion mass at a time.
- QitTOF enables fast TOF detection compared to IT MS only.
- QitTOF enables mass-selective analysis by MS-MS or MSn analysis compared with TOFMS only.
These features translate into high levels of performance in terms of speed, sensitivity, and specificity.
The ionizer is a key component of any MS detection system. The QitTOF MS detector that we employ for explosives detection uses a glow discharge ionization (GDI) source that operates in negative ion mode for explosives. This source is based upon the GDI source developed by McLuckey and coworkers (14,15). Explosives are very electronegative compounds that readily attach electrons. Most other common compounds that are likely to be sampled in a portal system are less electronegative; hence, the ionization process provides a stage of selectivity in differentiating explosives compounds from common background. However, as portrayed in Figure 2, other compounds besides explosives can be ionized, and therefore it is important to have the capability to further differentiate explosives signals.
Figure 2: Comparison of ion mobility spectrometry (IMS) and mass spectrometry (MS) methods of detection in an overall high-speed screening system.
The QitTOF MS detection system has the properties of high resolution, high sensitivity, molecular weight identification, and potential for secondary confirmation by ion fragment analysis (MS-MS). The detector was designed for automated and unattended operation and for use by nontechnical personnel, which is an important operational characteristic for deploying MS systems in mainstream environments staffed by nontechnical users.
MS versus IMS
Figure 2 is a starting point for comparing MS and IMS detectors in an overall screening system. Following the sampling and concentration process described earlier, the thermally desorbed vapor is introduced to the analyzer. Both MS and IMS use ionization, and for explosives compounds the primary mode is negative ionization. This provides some selectivity because explosives tend to be much more electronegative than typical background compounds. IMS generally uses a radioactive 63Ni source to create a plasma of ions and charges to ionize neutral molecules. It is also necessary to add a reagent gas, such as methylene chloride, to enhance the ionization of explosives compounds. Our MS system uses GDI, which does not require a reagent gas and is therefore consumable free, which is a benefit when deploying detection systems in mainstream 24/7 environments.
The principal advantages of MS over IMS are higher resolution and molecular identification. Parent and fragment ions of explosives compounds span a range of about 300 atomic mass units (for example, PETN MW = 316, NH3 MW = 17). MS provides better than unit mass resolution, so the overall resolution R for detection is at least 300. If MS-MS is employed, then the resolving power can approach 300 squared (though not all fragment ion masses are equally probable, so the effective resolution is less than this). IMS is a method that also separates ions in time. Separation is based on the different speeds that ions drift through a viscous gas under the force of an electric field. The drift time depends on both molecular weight and size; it does not give a simple measure of molecular weight. IMS resolution, defined as the ratio of the total span of drift times for target ion signal and the ion signal width, rarely exceeds 30.
As shown in Figure 2, higher resolution greatly reduces the problem of interference due to overlapping signals from different compounds. The measure of molecular weight by MS is a very strong indicator of what the compound is, unlike the measure of mobility times by IMS. For example, TNT has a molecular weight of 227. The probability of another compound having that molecular weight is very small. However, to positively confirm that a detected signal at molecular weight 227 is actually TNT, the QitTOF MS technology can selectively fragment the molecular ion to determine its structure and definitively identify the detected compound. This secondary analysis provides an unprecedented level of specificity for positively detecting targeted compounds.
A recent report from the National Academy of Sciences assessed the relative performance of IMS and MS and concluded that the latter provided from 10–10,000 better resolution, which translates into improved accuracy in terms of probability of detection and false positive rates (16). The higher end of this resolution range represents high-resolution instruments and MS-MS instruments.
Mass Spectral Signatures
The key to an effective trace explosives detection system is the simultaneous detectability of a broad range of compounds at trace levels in the presence of background compounds at much higher abundance. Achieving this capability is aided by a selective ionization process such as the GDI negative ion mode that maximizes the ion signal of explosives while minimizing the ion signal from the more abundant background compounds. Furthermore, it is essential that each compound have distinct and highly resolved molecular signatures to enable positive identification and differentiation from potential interferents. Figure 3 shows negative ion GDI/QitTOF mass spectra of common explosives. These compounds are observed to have distinct mass spectral signatures. Because the spectral signals are very sharp, the probability of overlap with other compounds, such as background compounds, is very low relative to lower resolution detectors such as IMS. Nitroaromatic explosives (such as TNT and DNT) generally give molecular ion signal. Nitramines (RDX and HMX) give distinct peaks at m/z of 176, 129, and 102, but not at the molecular ion. Nitrate esters give the least distinct spectral signatures due to extensive dissociative electron attachment to give a predominant NO3– ion at m/z 62. PETN leads to detectable signal at m/z 242 under optimized conditions to give the potential to distinguish this nitrate ester.

Figure 3: MS spectral signatures for selected explosives. Each compound gives a unique spectrum. Nitrate esters all have a common peak at m/z 62.
The spectra in Figure 3 were recorded from headspace vapor either at room temperature (TNT, PETN) or elevated temperature (about 50 °C for RDX). For TNT this corresponds to a saturated headspace vapor pressure of less than 10 ppb. At these levels strong signal is observed with relatively weak signal from room air. Explosives compounds that have been detected by the MS detector with high sensitivity include TNT, ADNT, DNT, TNB, DNB, DMNB, RDX, HMX, EGDN, NG, PETN, Tetryl, HMTD, AN, and UN.
Other MS Analyzers Used for Explosives Detection
Detecting explosives in complex environments requires very sensitive and specific analyzers. Although MS offers excellent detection limits and resolutions (sensitivity and specificity), general-purpose laboratory instrumentation is not ideally suited for detecting trace levels of explosives in the presence of large abundances of potential interferent compounds. Furthermore, MS traditionally has required a skilled operator not only to acquire data, but also to interpret it. MS developments have tended to focus on the performance issues and less so on the operational issues.
We review MS developments centering on achieving a trace detector for early warning and first response as opposed to an analytical instrument for postanalysis of a suspected event. A key strategy has been to focus on ionization methods that preferentially ionize explosives relative to typical background compounds. McLuckey and coworkers (14,15,17) developed an atmospheric sampling glow discharge ionization (ASGDI) source that could be operated in negative ion mode to achieve high sensitivity and specificity for detection of explosives (as also mentioned earlier). They demonstrated coupling it to quadrupole and ion trap MS systems and achieved sensitivities of < 10 pptv (and < 10 pg) for volatized TNT and RDX (without preconcentration), as well as demonstrated sensitive detection for a wide variety of other explosives compounds. The ASGDI source operates at about 1 torr, which is sufficiently high pressure to obtain reasonable densities of explosives, yet low enough to minimize deleterious effects due to ion–molecule reactions. The ASGDI source is the basis of the GDI source used in the MS explosives detection systems described here. Atmospheric pressure ionization (API) sources, particularly chemical ionization using discharge methods, have been adapted by us and other groups to explosives detection and have achieved exceptional sensitivity. Lee and colleagues (18) reported a detectable signal for TNT of 10 fg using an API/TOFMS instrument. Work by a SCIEX/British Aerospace team (19,20) demonstrated detection limits on the order of 1 pg for TNT, RDX, and PETN for an API–triple-quadrupole MS system in a program called CONDOR. However, API sources, which are also used in IMS, are prone to ion–molecule reactions that can deplete the signal for explosives ions depending on the abundance of certain background compounds.
More sophisticated MS configurations have been demonstrated for selective explosives ionization and detection, taking advantage of the high electron attachment cross-section for explosives. Chutjian and coworkers (21,22) developed the reversal electron attachment detection (READ) method, which relies on slowing and reversing an electron beam in a reflectron device. The electron energy at the turning point is close to zero where the electron capture ratio is greatest. Laramie and coworkers (23) developed the electron-capture negative ion MS (ECNIMS) system. This system varies the electron energy, which enables differentiation of different types of explosives by monitoring the electron energies at which the dissociated NO2– ion appears. Because the READ and ECNIMS systems employ ionization in the high vacuum region of the MS, sensitivity is compromised by the low molecular densities in the ionization region.
Other Applications
ETD detectors using IMS are ubiquitous in airports, with over 10,000 units purchased for U.S. airports and other security venues alone since 9/11. These detectors use cloth swipes to collect samples as described earlier. Recently, Hitachi introduced an MS version of the ETD detector using the swipe method. Little data is available at this time.
Another novel application of MS is the screening of boarding passes for explosives (and narcotics) (24). The MS detector in that case uses atmospheric pressure chemical ionization (APCI) and a triple-quadrupole MS analyzer operating in MS-MS mode. Detection is made by monitoring parent and characteristic fragment ions (those that differ in mass by the NO2 or NO fragment). The boarding pass screener was shown to operate at a throughput rate of up to 1000 boarding passes per hour and was able to detect between 10 and 50 pg of explosives residue from the surface of the card, depending on the compound monitored.
Interesting new technologies that can ionize substances directly on surfaces offer potential new opportunities for security screening of explosives. These include DART (direct analysis in real time, JEOL, Peabody, Massachusetts) (25) and DESI (desorption electrospray ionization) (26). The basis of DART is that a flowing afterglow discharge acts to thermally desorb and ionize compounds off of surfaces that can be analyzed by MS. DESI works by depositing a stream of ionized liquid onto a surface that causes charge transfer to surface compounds that desorb from the surface and can be analyzed by MS.
Summary and Conclusions
MS is often referred to as the gold standard for molecular detection and identification. Traditionally a laboratory-based research tool, MS is now being developed for automated operation in rugged environments by technically unskilled operators. It is therefore inevitable that MS will find a major role in security applications, particularly where detection accuracy outweighs the requirements for size and cost. The determining factor regarding whether to deploy an IMS detector or an MS detector depends on application. When inexpensive, handheld analyzers are required, IMS is the best choice. For stationary applications where compactness is less important, the superior performance of MS offers compelling benefits.
Acknowledgments
We acknowledge the many people at Syagen, too numerous to list, who have contributed to the explosives detection project. We also acknowledge our collaborators at Sandia National Laboratories for their critical contributions to the early development of our MS portal developments. Finally, we greatly appreciate the support given to us over the years by TSA and DHS.
Jack Syage and Karl A. Hanold are with Syagen Technology, Inc., Tustin, California.
References
(1) K.J. Hart, M.B. Wise, W.H. Griest, and S.A. Lammert, Field Anal. Chem. Tech. 4, 93–110 (2000).
(2) J.A. Syage, S-S Cai, J. Li, and M.D. Evans, Anal. Chem. 78, 2967–2976 (2006).
(3) J.A. Syage and K.A. Hanold, in Trace Chemical Sensing of Explosives, R.L. Woodfin, Ed. (Wiley, New York, 2007), Chap. 11, p. 219.
(4) J. Yinon, Mass Spectrom. Rev. 10, 179 (1991).
(5) D.S. Moore, Rev. Sci. Instrum. 75, 2499–2512 (2004).
(6) G.E. Spangler and R.A. Miller, Int. J. Mass Spectrom. 214, 95–104 (2002).
(7) B.A. Buckles, Fed. Regist. 67, 20864 (2002).
(8) J.E. Parmeter, K.L. Linker, C.L. Rhykerd, and D.W. Hannum, Mass Spectrom. Rev. 10, 187 (1991).
(9) S.M. Michael, B.M. Chien, and D.M. Lubman, Anal. Chem. 65, 2614 (1993).
(10) S.M. Michael, B.M. Chien, and D.M. Lubman, Rev. Sci. Instrum. 63, 4277 (1992).
(11) J.A. Syage JA and M.A. Evans, Spectroscopy 16, 14 (2001).
(12) J.A. Syage, B.J. Nies, M.D. Evans, and K.A. Hanold, J. Am. Soc. Mass Spectrom. 12, 648 (2001).
(13) L. Ding, E. Kawatoh, K. Tanaka, A.J. Smith, and S. Kumashiro, Proc. SPIE, 3777, 144 (1999).
(14) K.G. Asano, D.E. Goeringer, and S.A. McLuckey, Anal. Chem. 67, 2739 (1995).
(15) S.A. McLuckey, G.L. Glish, K.G. Asano, and B.C. Grant, Anal. Chem. 60, 2220 (1988).
(16) National Research Council, "Opportunities to Improve Airport Passenger Screening with Mass Spectrometry" (National Academies Press, Washington, D.C., 2003).
(17) S.A. McLuckey, G.J. Van Berkel, D.E. Goeringer, and G.L. Glish, Anal. Chem. 66, 689A, 737A (1994).
(18) H.G. Lee, E.D. Lee, and M.L. Lee, Proc. Int. Symp. Explosives Detection Technol., 1st , edited by S.M. Khan, Atlantic City, FAA, 619 (1992).
(19) W.R. Stott, W.R. Davidson, and R. Sleeman, Proc. SPIE, 2092, 53 (1994).
(20) G. Bennett, R. Sleeman, W.R. Davidson, and W.R. Stott, Proc. SPIE, 2276, 363 (1994).
(21) S. Boumsellek, S.H. Alajajian, and A. Chutjian, J. Am. Soc. Mass Spectrom. 3, 243 (1992).
(22) S. Boumsellek and A. Chutjian, Anal. Chem. 64, 2096 (1992).
(23) J.A. Laramee, C.A. Kocher, and M.L. Deinzer, Anal. Chem. 64, 2316 (1992).
(24) R. Sleeman, S.L. Richards, W.R. Stott, W.R. Davidson, J.G. Luke, B.J. Keely, I. Fletcher, and A. Burton, Proc. of the 50th ASMS Conf. on Mass Spectrom. and Allied Topics, Orlando, FL (2002).
(25) R.B. Cody, J.A. Laramee, and H.D. Durst, Anal. Chem. 77, 2297-2302 (2005).
(26) I. Cotte-Rodriguez, Z. Takats, N. Talaty, H. Chen, and R.G. Cooks, Anal. Chem. 77, 6755-6764 (2005).

High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
The Fundamental Role of Advanced Hyphenated Techniques in Lithium-Ion Battery Research
December 4th 2024Spectroscopy spoke with Uwe Karst, a full professor at the University of Münster in the Institute of Inorganic and Analytical Chemistry, to discuss his research on hyphenated analytical techniques in battery research.
Mass Spectrometry for Forensic Analysis: An Interview with Glen Jackson
November 27th 2024As part of “The Future of Forensic Analysis” content series, Spectroscopy sat down with Glen P. Jackson of West Virginia University to talk about the historical development of mass spectrometry in forensic analysis.
Detecting Cancer Biomarkers in Canines: An Interview with Landulfo Silveira Jr.
November 5th 2024Spectroscopy sat down with Landulfo Silveira Jr. of Universidade Anhembi Morumbi-UAM and Center for Innovation, Technology and Education-CITÉ (São Paulo, Brazil) to talk about his team’s latest research using Raman spectroscopy to detect biomarkers of cancer in canine sera.