Distinguishing Structural Isomers: Mono- and Disubstituted Benzene Rings
Spectroscopy
Following up on the last installment, we examine the infrared spectra of mono- and di-substituted benzene rings. We will examine numerous example spectra and learn how the position of C-H wagging peaks, and the presence or absence of a ring-bending peak, allow one to distinguish between mono-, ortho-, meta-, and para-substituted rings most of the time.
Following up on the last installment, we examine the infrared (IR) spectra of mono- and disubstituted benzene rings. We examine numerous example spectra and learn how the position of C-H wagging peaks, and the presence or absence of a ring-bending peak, allow one to distinguish between mono-, ortho-, meta-, and para-substituted rings most of the time.
Structural isomers are molecules that have the same chemical formula but different chemical structures. Therefore, these molecules have different chemical properties such as boiling points and reactivities. Structural isomers have the same mass so they are difficult to distinguish by mass spectrometry. However, infrared (IR) spectroscopy, where the peak positions are determined by the atomic masses and bond strengths of the functional group undergoing vibration (1), can distinguish between structural isomers because each structural isomer has a unique set of masses and bond strengths.
In the last installment (2), I introduced the topic of aromatic bonding and aromatic rings, and we looked at the spectrum of the prototypical aromatic ring benzene in detail. In this installment, we expand our knowledge of the IR spectroscopy of aromatic rings by looking at the spectra of mono- and disubstituted benzene rings. There are three ways of arranging two substituents around a benzene ring, meaning that disubstituted rings have three structural isomers, hence the need for a discussion of structural isomers and substituted benzene rings in one article.
Structures of Mono- and Disubstituted Benzene Rings
Benzene has six hydrogens and each of them can be replaced with a substituent, meaning a benzene ring can have up to six substituents. However, most of the substituted benzene rings you will come across will have one or two substituents, referred to as mono- and disubstituted benzene rings, respectively. Figure 1 shows the chemical structures of these types of rings.
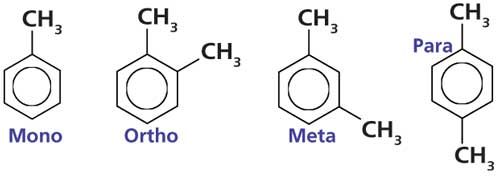
Figure 1: The chemical structures of mono-, ortho-, meta-, and para-substituted benzene rings, illustrated using toluene and the xylene family of molecules.
The monosubstituted molecule in Figure 1 is properly called methylbenzene, but most of us know it as toluene. As you can see, toluene simply consists of a methyl group attached to a benzene ring.
The three structural isomers of disubstituted benzene rings are named ortho-, meta-, and para-. Their structures are illustrated using the xylenes (or dimethylbenzenes) in Figure 1. Ortho-xylene has two methyl groups on adjacent carbons. Meta-xylene has two methyl groups separated by one carbon. In para-xylene the two substituents are across the ring from each other and there are two carbons between the methyl groups. I remember these substitution patterns by thinking of the letters OMP and recalling that the two substituents get further apart as one goes from ortho- to meta- to para-. To remember the letters OMP I use a silly chemical mnemonic like “Oh My Palladium.”
The infrared spectrum of toluene is shown in Figure 2. Toluene contains unsaturated carbons in its benzene ring and a saturated carbon in its methyl group, making it a “mixed molecule.” Recall (3) that unsaturated carbons generally have C-H stretching peaks above 3000 cm-1, whereas saturated carbons have C-H stretches below 3000 cm-1. Toluene conforms to this pattern with C-H stretches above and below 3000 cm-1 as denoted by the letter A in Figure 2.
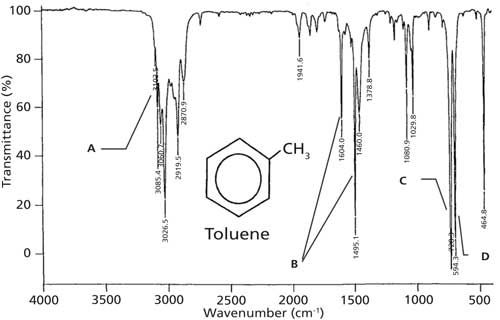
Figure 2: The IR spectrum of toluene, measured as a capillary thin film.
Recall that ring modes are from the stretching of the carbon-carbon bonds in the benzene ring, that they vary in number and intensity, and fall between 1620 and 1400 cm-1 (2). Toluene’s ring modes appear at 1604 and 1495 cm-1 as denoted by the letter B in the figure. The C-H wag, the most intense band in the spectrum, is seen at 728 cm-1 as denoted by the letter C. Previously I stated (2) that the C-H wags of benzene rings generally fall from 1000 to 700 cm-1. However, we can be more specific with this peak’s range. Benzene ring C-H wagging peak positions are sensitive to substitution pattern. For monosubstituted benzene rings such as toluene the C-H wagging peak falls between 770 and 710 cm-1.
The second most intense peak in Figure 2, labeled D at 594 cm-1, is new to us. The vibration giving rise to this peak is a ring bend. Imagine the carbon–carbon bonds in the molecule’s benzene ring bending above and below the plane of the molecule. The beauty of this peak is that depending upon a benzene ring’s substitution pattern it is both present and intense as seen in Figure 2, or completely absent. The presence or absence of this peak is determined by the symmetry of the substitution pattern, dµ/dx is either large or zero depending on whether the molecule is mono-, ortho-, meta-, or para-substituted. When it does appear, this ring bending peak falls at 690 ± 10 cm-1. The key then to distinguishing a monosubstituted benzene ring is a C-H wag between 770 and 710 cm-1 and the presence of a ring bend near 690 cm-1. As we will see, it is the position of the C-H wag and the presence or absence of the ring bending peak together that can be used to distinguish mono- and disubstituted benzene rings from each other.
The infrared spectrum of ortho-xylene is shown in Figure 3. Because this molecule has saturated and unsaturated carbons present, there are C-H stretching peaks above and below 3000 cm-1, as denoted by the A label. The ring modes are at 1604 and 1495 cm-1, which are labeled B. These ring-mode peak positions are identical to toluene, which is why ring modes should not be used to determine the substitution pattern around a benzene ring. The C-H wag in Figure 3 appears at 742 cm-1 as denoted by the letter C. For ortho-substituted rings in general this peak falls between 770 and 735 cm-1. Note that there is no ring bending peak near 690 cm-1 in this spectrum, as is typical of ortho-substituted rings. Thus, there is only one diagnostic peak for ortho-substituted rings, the C-H wag.
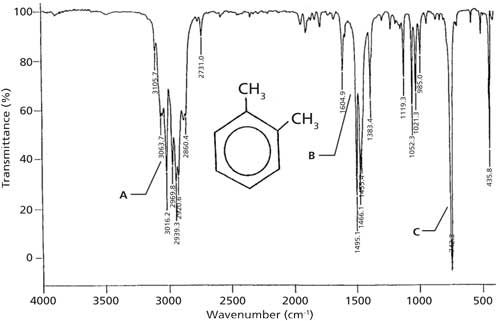
Figure 3: The IR spectrum of ortho-xylene, measured as a capillary thin film.
In theory, one could confuse the spectra of mono- and ortho-substituted rings because their C-H wagging peak ranges overlap. However, the ring bend is clearly present in mono-spectra and absent from ortho-spectra, meaning that these two are actually easy to distinguish from each other.
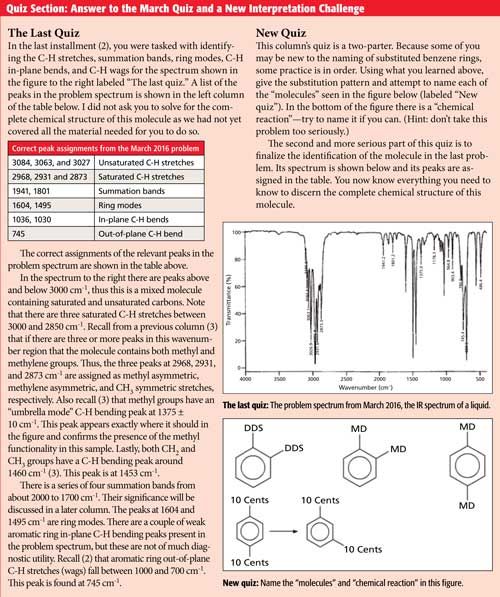
The IR spectrum of meta-xylene is shown in Figure 4. Since meta-xylene is a mixed molecule, there are C-H stretches above and below 3000 cm-1 (labeled A). There are ring modes at 1612, 1591, and 1491 cm-1 denoted B, and the C-H wag is at 768 cm-1 labeled with a C. For meta-substituted benzene rings in general, the C-H wag falls from 810 to 750 cm-1. The second most intense peak in the spectrum at 691 cm-1, denoted with a D, is a ring bend. Ring bends for meta-substituted benzene rings appear in the same range as for monosubstituted rings, 690 ± 10 cm-1. Thus, the diagnostic peaks for meta-substituted rings are the presence of a C-H wagging peak from 810 to 750 cm-1 and the ring bending peak near 690 cm-1.
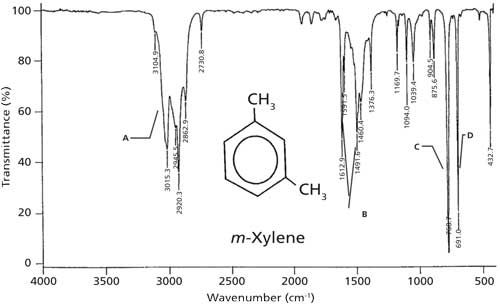
Figure 4: The IR spectrum of meta-xylene, measured as a capillary thin film.
The infrared spectrum of para-xylene is shown in Figure 5. As expected there are C-H stretches above and below 3000 cm-1 in this spectrum labeled A. There is a strong ring-mode peak at 1516 cm-1 denoted with a B, and the C-H wag is at 795 cm-1 as pointed out with a C. For para-substituted benzene rings, generally the C-H wag falls between 860 and 790 cm-1. Note the absence of a ring bend, so the only diagnostic feature for para-substituted rings is a C-H wagging peak.
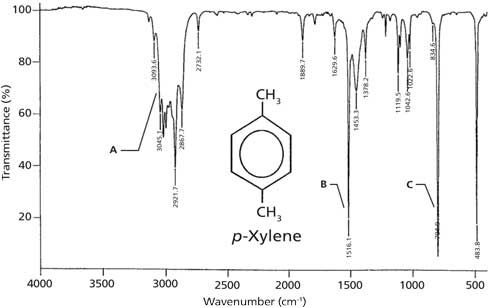
Figure 5: The IR spectrum of para-xylene, measured as a capillary thin film.
We have seen from four examples that the position of the C-H wag and the presence or absence of the ring bending peak together can be used to distinguish mono- and disubstituted benzene rings from each other. This information is summarized in Table I. If you look closely at this table you will notice that if the C-H wag falls between 770 and 750 cm-1 and there is a ring bend present then the molecule could be mono- or meta-substituted. There is a way to distinguish between these molecules in this situation using other information in the spectrum, which will be discussed in the next column.

References
- B.C. Smith, Spectroscopy 30(1),16–23 (2015).
- B.C. Smith, Spectroscopy31(3), 34–37 (2016).
- B.C. Smith, Spectroscopy 30(4), 18–23 (2015).

Brian C. Smith, PhD, is a Senior Infrared Product Specialist for PerkinElmer, based in San Jose, California. Before joining PerkinElmer, he ran his own FT-IR training and consulting business for more than 20 years, and taught thousands of people around the world how to improve their FT-IR analyses and interpret infrared spectra. Dr. Smith has written three books on infrared spectroscopy: Fundamentals of FTIR and Infrared Spectral Interpretation, both published by CRC Press, and Quantitative Spectroscopy: Theory and Practice published by Academic Press. He has published a number of papers in peer-reviewed journals and is a co-inventor on a patent for an FT-IR method to monitor dust exposure in coal mines.

The Future of Neurodegenerative Disease Research and the Role of IR Imaging
May 21st 2025In the final part of this three-part interview, Ayanjeet Ghosh of the University of Alabama and Rohit Bhargava of the University of Illinois Urbana-Champaign talk about the key performance metrics they used to evaluate their model, and what the future of neurodegenerative disease research looks like.
Describing Their Two-Step Neural Model: An Interview with Ayanjeet Ghosh and Rohit Bhargava
May 20th 2025In the second part of this three-part interview, Ayanjeet Ghosh of the University of Alabama and Rohit Bhargava of the University of Illinois Urbana-Champaign discuss how machine learning (ML) is used in data analysis and go into more detail about the model they developed in their study.
AI and Infrared Light Team Up to Advance Soil Carbon Monitoring
May 19th 2025A team of international researchers has developed a faster, more accurate method to analyze soil carbon fractions using mid-infrared spectroscopy and deep learning. Their approach preserves the chemical balance of soil organic carbon components, paving the way for improved climate models and sustainable land management.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)