The Dynamic World of X-ray Fluorescence
From the field to the synchrotron, XRF is expanding its power and scope.
Many people think X-ray analysis, including X-ray fluorescence (XRF) spectrometry, is a mature technology with limited sensitivity, useful primarily for low-resolution industry process applications. They should think again. Over the past two decades, XRF has evolved and matured, but not into a boring old age. Rather, its current capabilities, including sharper optics and ever-improving handheld devices, have led to use in new application areas and expanded its use in areas where it previously penetrated only nominally. At the same time, cutting-edge research is ongoing in areas such as two-dimensional (2D) and three-dimensional (3D) X-ray mapping using micro X-ray fluorescence (µXRF), as well as in synchrotron radiation–based µXRF and micro X-ray absorption near edge structure (XANES). That work at synchrotrons is enabling fascinating research in fields such as cellular biology, nanotechnology, and cultural heritage, and at the same time is prompting further investigation into what can be achieved with XRF in the laboratory and the field.
Instrument Developments: Detectors, Optics, and Computing Power
XRF's current higher status in the field of spectroscopy results from important developments in several enabling technologies over the past two decades that led to critical improvements in the capabilities of modern XRF instruments.
First, the development in the late 1980s of the room-temperature silicon p-i-n diode detector made it possible to replace the large, gas-filled proportional counter with a thimble-sized device with excellent energy resolution. This led to wider use of sophisticated quantification algorithms and at the same time started a gradual change in the form factors and the miniaturization of energy-dispersive X-ray fluorescence (EDXRF) systems.
In addition, broadband polycapillary optics, developed in the mid-to-late 1990s by XOS (East Greenbush, New York), greatly improved the spatial resolution and signal-to-noise ratio of XRF generally, and enabled the field of µXRF more specifically. More recently, XOS has introduced narrowband doubly curved crystal (DCC) optics, which are designed to greatly reduce background noise and are useful for applications examining a narrow range of elements.
The other major change in this period was the development of the miniature X-ray tube for use in EDXRF instruments. Earlier EDXRF instruments designed for field use relied on radioactive isotopes as the source of exciting radiation. Although such sources were small, they were not as efficient as X-ray tubes. Also, the radioisotopes could not be turned on and off, and strict safety regulations made transporting radioisotope-based instruments complicated.
With the availability of miniature, battery powered X-ray tubes, XRF units suddenly became much more mobile, and over the past decade the use of mobile and specifically of handheld units has skyrocketed. Today, the ongoing adaptation of handheld units for specialized applications is the fastest growing area in XRF.
Lastly, XRF analysis, just like most aspects of science today, has benefited greatly from increased computational capability. Calculations and calibration are better and faster, and the modeling that underlies the interpretation of XRF spectra has improved significantly.
"Recent improvements in computational capability are translating into new ways to analyze data and new ways to use instrumentation," says Tim Elam, senior physicist at the Applied Physics Laboratory at the University of Washington (Seattle, Washington) and co-chair of the Denver X-ray Conference. "I think we've just scratched the surface of what those capabilities will enable us to do."
As a result of all of these developments in XRF technology, the use of XRF is expanding, although the growth areas for industrial applications vary from one part of the world to another. Quality control process applications for heavy industries like cement and steel manufacturing are growing the most in developing countries. In industrialized nations, meanwhile, XRF is used increasingly for the analysis of more-complex samples.
"U.S. factories increasingly have to make materials that are more specialized and more demanding to make," says Alexander Seyfarth, XRF product manager for North America at Bruker AXS (Madison, Wisconsin). "That means that XRF has to be able to do more complicated analysis, such as analyzing new battery types, solar cells, or high performance polymers that have low tolerances."
Another growth area in industrialized countries is analytical testing done to comply with environmental and safety regulations. Regulations such as Europe's Regulations of Hazardous Substances (RoHS) Directive have generated a lot of XRF use for analyses such as detecting lead in plastics, toys, and other materials.
Testing for lead also highlights the effectiveness of modern XRF systems as a screening tool, says Stan Piorek, director of applied research for portable XRF analyzers at Thermo Fisher Scientific (Billerica, Massachusetts).
"By using XRF instead of a destructive laboratory technique, you can test 100% of the product instead of doing random sampling," he points out. "As a result, your compliance with safety regulations is better overall, and you spend less time and money doing it."
And in a screening application, the fact that XRF detection limits are often not as low as those of inductively coupled plasma (ICP) or other atomic spectroscopy techniques is not a problem. In cases where the sensitivity of XRF is insufficient to be sure if a limit has been passed, one sends those samples off to the laboratory. "XRF is great for sorting things so you can spend your effort on those that need it," Piorek concludes.
Watch Out ICP, Here Comes X-ray
But one trend is common everywhere in the world: XRF is entering new fields of application and expanding in analysis areas formerly considered the domain of other types of atomic spectroscopy.
"With the increased capabilities and decreasing cost of XRF instrumentation, XRF has crossed a threshold," says Elam. "Measurements that traditionally were made using one of the atomic emission spectroscopies, such as ICP, ICP-mass spectrometry (MS), or atomic absorption, have begun shifting to XRF."
Testing for impurities in pharmaceuticals, he says, is a case in point. Traditionally, such an analysis would be done by digesting the sample and analyzing it with one of the atomic emission methods. "But now, it seems to be somewhat more favorable to do it with XRF, partly because of improvements in XRF instrumentation and partly because XRF can measure solid samples directly, without the digestion step," he says. "That is a huge improvement in throughput and ease of use."
Maggie Loubser, group chief chemist at PPC Cement in Cleveland, South Africa, also sees XRF taking on roles previously reserved for other methods. She recently validated an XRF method for the trace-element analysis her company must carry out to comply with environmental legislation. In the past, that work was done with ICP. "It's not ultratrace analysis; we basically want to know if there is less than 100 ppm of a heavy metal in our materials," she says. "XRF is very effective in these ranges, and relatively cheap compared to ICP, and also of course the man-hour input is a lot less."
Instruments Tailored to a Single Purpose
But much of the expansion in XRF use has been a result of vendors developing instruments to solve very specific problems. This is particularly true for the handheld instruments.
"Today, handheld units are not sold as analyzers, they are sold as ready-made solutions," says Seyfarth. "You are buying a mining unit, a soil unit, or a scrap metal analyzer." And although this may sound like just clever marketing, the instruments are indeed highly tailored to solve specific analytical problems. Vendors are building XRF instruments that can detect and quantify lower concentration ranges for specific elements, for example, by developing specific modifications of the technology.
The optics manufacturer XOS, which now also sells mobile XRF units, is among those who have taken the approach of adapting instruments to particular industry needs. "We design single-purpose instruments based on economic drivers for a particular industry," says David Gibson, president of XOS. "For example, we have an instrument for measuring sulfur in petroleum, and that's all that instrument does. It can't measure any other element. But it is really well tuned for measuring sulfur in petroleum, all the way from crude to fine distillates." That fine-tuning meant decreasing the detection limits for that element to concentrations as low as 0.15 ppm in the company's latest version of the instrument.
This kind of specialization is good for XRF as a whole, says Gibson. "It opens up the market dramatically," he says. "You make it possible to use XRF where you couldn't conceive of having a broad purpose, complicated analytical instrument."
And like many of the specialized handheld XRF instruments on the market today, this one was designed to be used by operators who are not spectroscopists, and in many cases aren't even scientists. "We designed this instrument to be used by someone with a high school education and 15 minutes of training from the person coming off the preceding shift," says Gibson.
The Power of Combining XRF with XRD
Piorek thinks the expansion of XRF will expand further as users increasingly combine XRF with other techniques, such as X-ray diffraction (XRD), because the two techniques are very complementary. He cites the example of inline process control in steel manufacturing. "XRF can give you elemental composition, but maybe you also want to know what crystalline form it's in, so for that, you would use XRD," he says.
Loubser says this combination is already in use in her company's cement plants. "X-ray powder diffraction for many years was an academic tool, not really used in product and process control very much, but with the advance of more user-friendly software, more and more it gets incorporated," she says. "We, for instance, in many of our plants now use XRF analysis in conjunction with X-ray diffraction analysis. So we don't just look at the elements in the process, we also look at the phases that are formed. The combined data give you the full picture."
Beyond Routine Analysis: New Research in Industry, Space, and Art
But don't be fooled. XRF is not just for routine industry applications requiring less-sensitive detection. It is also being used for detailed analyses and to create 2D and 3D elemental images, or maps, for applications ranging from industry quality control to art conservation, and even to explore the viability of extraterrestrial life. In particular, XRF work being carried out at synchrotrons is enabling analyses that were not possible before.
Elemental Mapping with µXRF: The Power of Analytical Pictures
Another exciting area of development in XRF analysis is 2D and 3D elemental imaging, or mapping. 2D elemental mapping is done primarily with µXRF, in which the sample is moved under a spatially restricted X-ray beam. This approach provides elemental intensities as a function of position, and those elemental intensities can then be assembled into a map.
"An elemental map is a powerful way to convey elemental analysis of a sample," says George Havrilla, a research scientist at Los Alamos National Laboratory (Los Alamos, New Mexico). "They say a picture is worth a thousand words; I say a picture is worth a thousand analyses."
Various manufacturers have commercialized µXRF instruments for 2D elemental mapping, and the method is being applied to various fields. Havrilla's group has used this method to look at the solder composition in electronic circuits. European regulations now restrict the lead content of various materials, but in some cases, such as solders, a minimum lead content is needed to make them function properly.
"There's a problem with what they call 'tin whiskers' that form if you have too little lead in a solder," explains Havrilla. "If you are dealing with a satellite, where you are in a weightless environment, these whiskers could break off and short-circuit a line, for example." So he has been using µXRF imaging to verify the composition of solders to ensure they have the required minimum lead content.
In one example, Havrilla examined a single point and confirmed it contained lead. But when he mapped the entire solder, he found pure tin in the edge of the solder, where it connected two parts. "This case proves the value and the power of being able to collect images," he says. "Elemental mapping enabled me to find something that a single point did not capture."
3D Mapping with Confocal XRF: New Ideas about Ancient Fossils
But as powerful as 2D mapping is, what really excites Havrilla is 3D elemental mapping with confocal XRF spectrometry. "With confocal XRF, every time we put a new sample in, it's like opening a new box on Christmas morning," he says. "We don't know what we are going to find."
Confocal XRF uses two monolithic polycapillaries; one focuses the X-rays to a focal spot and the second optic is oriented on the detector side for emission collection from the excitation focal spot. This setup makes it possible to scan the sample in the x, y, and z directions and produce a 3D elemental distribution. Havrilla developed the method, initially done at synchrotrons, and built the first confocal XRF instrument with XOS using the company's polycapillary optics.
Havrilla and his team at Los Alamos have used this method for a variety of applications, such as examining the density distribution of aerogel materials, which are targets for fusion research. Havrilla also has used the technique to examine the 3D distribution of the elements in fossils, a study initiated by his long-time colleague Robert Morton of Conoco-Phillips (Houston, Texas).
These maps have provided new insights into ancient creatures. In one study of a 650-million-year-old fossil, confocal XRF revealed no remnants of iron, but did find evidence of chromium (Figure 1). That led the archeologists to think that the organism might have had a chromium-based circulatory system, rather than one based on heme. "Results like that offer insights into the creature that formed that fossil, that you can't glean from just your eyeball," says Havrilla.
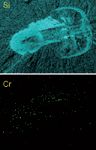
Figure 1: Confocal XRF maps of a fossil showing the positions of silicon (upper image) and chromium (lower image).
Exploring Life on Mars
This November, NASA's Mars Science Laboratory will be launched on a mission to Mars that will deploy a new rover, called "Curiosity." Curiosity will include several analytical instruments, including "CheMin," which uses XRD and also has limited XRF capabilities.
The goal of CheMin and the other instruments on Curiosity is to assess a local region on the Mars surface as a potential habitat for life. "We'll be looking for minerals that would suggest that water was present or that the environment was clement at some time in the past, meaning that it was less than about 100 °C and had materials that could have supported life," explains David Blake, a research scientist in the Exobiology Branch of NASA Ames Research Center (Moffett Field, California), who developed CheMin.
Although the original CheMin project included full XRD and XRF capabilities, the XRF capabilities were "downscoped" from the original plan because of difficulties making the XRF components, particularly the diode detector, robust enough for space. "Space instruments are pretty strange animals," Blake explains. "They have to work between -70 and +70 °C, and they have to take launch loads, the coldness of space, and vacuum, and we just got to the point where it would have been too difficult to do what we wanted to do with XRF."
Nevertheless, he hopes to include XRF in future missions. "The combination of XRF and XRD would really give you something," he says. "We were really excited early on about doing the linear equations to combine XRF and XRD data, to really get quality answers."
Synchrotron Radiation–Based XRF: Revealing the Secrets of Famous Paintings
Other XRF specialists are using µXRF and other X-ray techniques at synchrotrons to fine-tune their analyses even further for applications as diverse as cellular biology and cultural heritage.
Koen Janssens, professor of chemistry at the University of Antwerp (Belgium), is using synchrotron radiation–based XRF to analyze historic works of art. In a recent study, he used various spectroscopic techniques, including several X-ray methods, to reveal the process by which the yellow paint in some of Vincent Van Gogh's paintings darkened over time.
By applying a combination of synchrotron-radiation µXRF and µXANES, Janssens was able to determine that the darkening was a result of the hexavalent chromium, or chromium(VI), in lead chromate yellow paint, having been reduced to chromium(III). That reduction was induced by lead sulfate in the white paint Van Gogh mixed with the lead chromate to get a lighter shade of yellow.
Janssens was dealing with tiny paint samples that came off the paintings as a result of damage or restoration efforts and that had only a very thin layer of discolored paint on the surface (~2–3 µm thick), "For that kind of analysis, you need a method that allows you to analyze each layer separately," he says. So he used synchrotron radiation–based µXRF to get a very small X-ray beam, around 0.5–1 µm in diameter. X-ray beams from normal laboratory instruments generally produce beams around 10–20 µm in diameter.
In this study, a synchrotron was also used for the XANES measurements that made it possible to map the different oxidation states of chromium across the samples. XANES involves varying the energy of the X-rays that irradiate the sample, to take advantage of the fact that there is a very small difference in the binding energy of the K-shell electrons of different oxidation states of a single element. By determining the exact energy that is required to remove K-shell electrons from an atom, one can derive information about its oxidation state. Using a synchrotron allowed Janssens to get highly monochromatic primary X-rays and thus carefully select the primary energies needed to distinguish between chromium(VI) and chromium(III).
"We can capitalize on that small difference, which is just a few electron volts difference, by carefully choosing the energy we use to record the XRF maps with," Janssen says. "If you shoot at material which contains a mixture of chromium(III) and chromium(VI) and we use the right energy, it will only be the chromium(VI) that will absorb the radiation and will emit chromium fluorescence X-rays." So by carefully selecting the energy, Janssens was able to create chromium(VI)- and chromium(III)-specific maps.
From the Synchrotron to the Laboratory
The work Janssens and others are doing with synchrotron-based X-ray analysis has value for the broader field of X-ray fluorescence. "The movement from the work at synchrotrons into the laboratory is twofold," says Elam. "It gets people excited about what they might be able to do in the lab, and it also pushes the development of new optics and new techniques, which are directly applicable to and improve what one can do in the lab."
Summary
The field of X-ray fluorescence is dynamic and growing. As a result of significant improvements that began in the 1980s and continue today, XRF instrumentation is more capable than ever. Those capabilities, combined with lower prices and ongoing adaptation to specific application needs, are generating XRF growth in fields that formerly were reserved for the more modern methods of atomic spectroscopy, and in other fields where previously spectroscopy was not used at all. At the same time, specialized methods such as µXRF and synchrotron-based XRF and related X-ray techniques are enabling new and exciting exploration of a wide range of fields, like cellular biology, the study of cultural heritage, and even the search for extraterrestrial life.
George Havrilla sums up the excitement that he and other leaders in this field seem to share. "I am amazed at the range of questions that XRF can help answer, particularly as we reduce the spatial resolution of the X-ray probes," he says.
Tim Elam agrees. "I am glad to be working in this field," he says. "It is an exciting place to be."
Laura Bush is the editorial director of Spectroscopy and LCGC, (732) 346-3020, lbush@advanstar.com.

New Study Reveals Insights into Phenol’s Behavior in Ice
April 16th 2025A new study published in Spectrochimica Acta Part A by Dominik Heger and colleagues at Masaryk University reveals that phenol's photophysical properties change significantly when frozen, potentially enabling its breakdown by sunlight in icy environments.
Tracking Molecular Transport in Chromatographic Particles with Single-Molecule Fluorescence Imaging
May 18th 2012An interview with Justin Cooper, winner of a 2011 FACSS Innovation Award. Part of a new podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2012 ? the Great Scientific Exchange, the North American conference (39th Annual) of FACSS.
Advancing Corrosion Resistance in Additively Manufactured Titanium Alloys Through Heat Treatment
April 7th 2025Researchers have demonstrated that heat treatment significantly enhances the corrosion resistance of additively manufactured TC4 titanium alloy by transforming its microstructure, offering valuable insights for aerospace applications.
New Fluorescence Model Enhances Aflatoxin Detection in Vegetable Oils
March 12th 2025A research team from Nanjing University of Finance and Economics has developed a new analytical model using fluorescence spectroscopy and neural networks to improve the detection of aflatoxin B1 (AFB1) in vegetable oils. The model effectively restores AFB1’s intrinsic fluorescence by accounting for absorption and scattering interferences from oil matrices, enhancing the accuracy and efficiency for food safety testing.