Eponymous Mass Spectrometry: The Brubaker Prefilter and Kendrick Mass
An important method of recognition in the scientific community is to use an individual's name in a description of the contribution, known as an eponymous tribute. Mass spectrometry showcases a number of inventions named after the inventor. Here, we explain two: the Brubaker prefilter and Kendrick mass.

The time is ripe for further recognition of the breadth of analytical mass spectrometry. Other than awards, prizes, and fellowships, an alternative method of recognition in the community is to use an individual's name in a description of the contribution — an eponymous tribute. Eponymous terms enter into the literature independently of prize or award, and are often linked to a singular, specific, and timely achievement. Here, we highlight the Brubaker prefilter and Kendrick mass.
The 2013 annual meeting of the American Society for Mass Spectrometry (ASMS) concluded a few months ago. The continued growth of that conference, and the breadth of research and application in mass spectrometry (MS) evident there and described in other professional research conferences, reflects the vitality of modern MS. That growth seems to continue with few limits. The growth is catalyzed by a need for analysis in new application venues at better sensitivities, but growth is also supported by innovation in instrumentation and information processing. The 2013 ASMS conference celebrated 100 years of MS, and Michael Gross gave a presentation "The First Fifty Years of MS: Building a Foundation." It is still an unattainable ideal to reliably forecast the future simply by examining the past, and truly significant advances often seem to appear from nowhere. As is true in most scientific research, MS develops predominantly through incremental improvements. We can generally predict such improvements. More singular revolutionary advances, still recognized over the perspective of years, should be recognized through research awards such as the Nobel Prize. A third type of advancement lies between the two, and is neither simply iterative (which belongs to everyone in a sense) or transformative (which is recognized by a singular prize such as the Nobel). These important achievements are recognized within the community by awards of professional organizations, but also through high citation in the literature of specific publications as well as the eponymous citation. Eponymous citations, interestingly enough, sometimes do not include a traditional citation and reference to a publication. Often, the use of a name stands alone. For example, in recent columns in this series, we have described the use of Girard's reagents in derivatization, Penning ionization, and the Faraday cup detector. Current scientific publications that use that method or those devices may not cite all the way back to the original publication, and may not include any citation at all. Using the name alone seems to suffice.
In some fields of science, such as organism biology, the naming rights for a newly discovered organism go to its discoverer, and the scientist's name can be part of the term eventually approved by scientific nomenclators. In astronomy, naming rights for celestial bodies may also link to the first discoverer, or organizations may provide recognition of past achievements through the use of names. Features on the Moon and Mars, for example, reflect significant past scientific achievements. The instruments still roving Mars are providing a large number of "naming opportunities," some of which seem to be tongue-in-cheek. In chemistry, organic chemists who develop a certain reaction often see their names associated with that reaction (1–3). Ion fragmentation reactions in MS can follow this tradition; the McLafferty rearrangement is a significant eponymous example. In instrumentation and engineering, devices and mechanical inventions are also often named after their inventors (4). Sometimes, even when the inventor shuns publicity (5), the device is so perfect for its application that it remains in use for decades. Thus, users encounter the Brannock device without ever knowing the name, perhaps, but the cognoscenti are familiar with its history.
Mass spectrometers are complex instruments, encompassing technologies from vacuum science, ion optics, materials science, electronics, information and computer science, and more. Instrumental MS showcases a number of inventions named after the inventor. We describe some here in this column. These represent eponymous terms that are, like the Brannock device, so widely used or are such an integral part of the science that they are referred to by name without citation or explanation. Table I includes a few eponymous terms that should be familiar to mass spectrometrists. In some cases, as for the several eponymous terms containing the name of A.O.C. Nier, the pioneering contributions have been described in honorific publications (6). The list in Table I is not comprehensive and does not include underlying eponymous terms in basic science such as Taylor cone, Fourier transform, Coulomb's law, Franck-Condon transitions, or the like. Some eponymous terms are in limited use or they may appear in the literature only a few times; these occurrences are both hard to discover and difficult to document. From the perspective of 50 or 100 years in laying the foundation for MS, there may be a few things that we have always known that we did not actually know until somebody pointed it out. The goal in this column is not to revisit some obscure contribution, but rather to provide an appreciation of contributions that support modern MS.
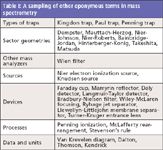
Table I: A sampling of other eponymous terms in mass spectrometry
Brubaker Prefilter
The basic concept of the quadrupole mass filter and the quadrupole ion trap was first disclosed in 1953; it was 36 years later that the contribution was recognized by the award of the 1989 Nobel Prize in Physics. Contrast that period of 36 years with the shortened period of only a few years between the awarding of the Nobel prize and discovery of deuterium and the neutron, as was described in a recent "Mass Spectrometry Forum" column in July 2013. By 1989, successful quadrupole-based commercial instrumentation had been on the market for almost 20 years. The rapid rise of gas chromatography coupled with mass spectrometry (GC–MS), and later liquid chromatography with mass spectrometry (LC–MS) can be linked directly to quadrupole devices. Quadrupole mass filters and quadrupole ion traps represented a fundamental change — they were smaller, cheaper, and their performance measures dovetailed with the needs of the hyphenated coupling.
The Nobel prize recognized the transformative accomplishment (7). Continuous design innovations over many years created a more capable and reliable instrument, and these are described in an overview by March (8), selected from among the many that are available. In concordance with the theme of personal developments in the advancement of mass spectrometry, Stafford's retrospective (9) discusses the development of the ion trap at one commercial vendor.
Let's consider the four-rod classical quadrupole mass filter. A key concept of this mass analyzer is its operation through application of radio frequency (rf) and direct current (DC) voltages to create "regions" of ion stability and instability within its structure. The term region applies to the physical volume subject to the electronic fields and gradients; each physical volume in the device is a certain distance from each of the four rods that make up the quadrupole mass filter, and a certain distance from either the source or the detector end, and the electric fields can be controlled. In simple terms, ions that pass through the filter from the source to the detector remain within stable regions. Those ions that enter regions of instability do not; they are ejected from the filter entirely or collide with the rods themselves. The quadrupole rods may be manufactured with different dimensions and the rods may be of various lengths. The applied fields vary in frequency and amplitude. Each of these factors influences the performance metrics in predictable ways. Given an entering ion with known kinetic energy, and a defined known trajectory, the performance of the quadrupole mass filter is predictable.
However, ions (plural) are an unruly lot. It is not one ion that needs to pass through the quadrupole, but hundreds of thousands of ions, created in an ion source with its own physical design and operational characteristics. Therefore, the population of ions exiting the source and entering the mass analyzer has a spread of ion kinetic energies and a range of ion trajectories, as well as a diversity of ion masses and charge states. The stable ions may represent just a small fraction of that overall population, which would limit the transmission of the filter and result in decreased sensitivity for the analysis. As in other mass analyzers used in mass spectrometry, we might use lenses, slits, or other sectors (such as an electric sector in a double focusing geometry instrument) to tailor the kinetic energies and trajectories, and pass a larger fraction of the ion population into the mass analyzer.
Or, in quadrupoles, we might use a Brubaker prefilter (sometimes called a Brubaker lens) situated in front of the mass-analyzing quadrupole. Wilson M. Brubaker was granted patent 3,129,327 (and later a related patent 3,371,204) for this device; the first patent was filed in December 1961 and granted in April 1964 (see Figure 1 for the first page of this granted patent). Brubaker worked for Consolidated Electrodynamics Corporation in Pasadena, California in the early 1960s. He was active in fundamental studies of the motions of ions in strong electric fields and in the development of small quadrupoles used to study the composition of the Earth's upper atmosphere. He also worked for Analog Technology Corporation and was the chief scientist for earth sciences at Teledyne Corporation. It was at this last position that Brubaker developed a miniature mass spectrometer (based on a quadrupole) for breath analysis for astronauts, which garnered a newspaper story on October 31, 1969. Brubaker's photograph that accompanied the article shows him posing with the sampling port of the mass spectrometer, and the photo is now available on eBay!
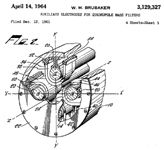
Figure 1: A figure from Brubaker’s original patent, showing three of the four rods in the prefilter. The ions move along the z direction from lower left to upper right, and into the main structure of the quadrupole mass filter.
A Brubaker prefilter (10) (Figure 2) consists of a set of four short, cylindrical electrodes (sometimes called stubbies) mounted colinearly and in front of the main quadrupole electrodes. Without the prefilter, ions from the source may not enter the quadrupole because of fringing fields at the front end, which are created by the exposed ends of the rods themselves. Transmission decreases because of this front-end loss. The Brubaker prefilter is driven with the same AC voltage (that is, the radio frequency voltage) applied to the main rods, and acts to guide ions in to the main quadrupole. It is an "rf-only" quadrupole that was later used in triple-quadrupole MS-MS instruments. The dynamics of these devices have been widely studied (11,12) and they serve several functions in the triple quadrupole instrument, depending on the pressure within the device. With the use of a Brubaker prefilter in a single-quadrupole instrument, the transmission of ions is greatly increased, although the increase is known to be mass-dependent. These devices are almost universally designed into quadrupoles. It is interesting that Brubaker's invention of the prefilter was a consequence of his need to maintain ion transmission in very small quadrupoles, such as what might be carried aboard the probes into the upper atmosphere or in spacecrafts. Transmission losses because of fringing fields are especially severe in these small devices. Nowadays, quadrupole mass filters can be microfabricated, with the prefilter and filter assembly only 3.2 cm in length. Wright and colleagues describe the incorporation of the Brubaker prefilter in miniature quadrupole mass filters (13).
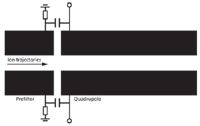
Figure 2: A simplified schematic (looking along the y-axis) of a Brubaker prefilter and the main quadrupole structure.
Kendrick Mass
As Table I shows, devices used in MS are often linked to the names of their inventors or designers. Modern MS is not only about the instrumentation, but also about the immense amounts of data generated by those instruments. From the first compilations of mass spectra in a three-ring binder from the American Petroleum Institute to the Eight Peak Index of Mass Spectral data (designed for searching of the most intense peaks in the mass spectrum) to larger modern databases, scientists have worried how to archive, present, and search mass spectral data. Mass spectral data are always at least two-dimensional (2D) (the m/z of an ion and its abundance). Time (as in information recorded with elution time in GC–MS or LC–MS) adds another dimension. Both higher resolution data and MS-MS data add dimensionality. A recent "Mass Spectrometry Forum" column (14) discussed the data management issue in MS. Large data sets can be mined for both their explicit first-order content and other meaningful secondary measures can sometimes be extracted. Insightful means of data presentation help tame the data glut. For example, time-resolved ion intensity plots for multiple-reaction-monitoring data highlight the underlying pattern. Three-dimensional (3D) color-coded plots for MS-MS data provide a portrait of mixture complexity and reactivity. Statistical evaluation of large amounts of data, or processing and display of data by computer, aid in presentation and interpretation. Oftentimes, these methods rely on the same fundamental perspectives in presenting data.
What if one changed the perspective? More than that, how about changing a really basic approach? In a previous installment of this column (15), we discussed the standard definition of the kilogram and the standard definition of the dalton. The consensus standards provide an underlying uniformity for our data and our discussions. With data processing, we can now easily shift standards and alter perspective. But 50 years ago, consider the value of such a new perspective (16):
The problems of computing, storing, and retrieving precise masses of the many combinations of elements likely to occur in the mass spectra of organic compounds are considerable. They can be significantly reduced by the adoption of a mass scale in which the mass of the CH2 radical is taken as 14.0000 mass units. The advantage of this scale is that ions differing by one or more CH2 groups have the same mass defect. The precise masses of a series of alkyl naphthalene parent peaks, for example, are 127.9195, 141.91 95, 155.91 95, etc. Because of the identical mass defects, the similar origin of these peaks is recognizable without reference to tables of masses.
That quote is from the abstract of a 1963 article (16) by Edward Kendrick, from the Analytical Research Division of Esso Research and Engineering. Kendrick confronted the reality that high resolution data (then measured at a resolving power of 5000) could be obtained for ions up to m/z 600 and concluded that "over one and a half million entries would be required to cover the ions up to mass 600. A mass scale with C12 = 14.00000 enables the same data — [that is], up to mass 600 — to be expressed in less than 100,000 entries. This reduction is a consequence of the same defect's being repeated at intervals of 14 mass units — [that is], one (CH2) group." It is not difficult to understand that a scientist at Esso would deal with a great many compounds that could differ by an integral number of methylene units (CH2). Given this challenge, the proposed approach and a shift in perspective was extraordinarily insightful. The eponymous term is the Kendrick mass scale, and the difference between the standard mass scale and that proposed is called the Kendrick mass defect. A mass unit called the kendrick (Ke) has also been used.
The original publication by Kendrick (16) provides the rationale for this change in perspective and contains multiple tables used to illustrate its usefulness. A short summary is provided here. In the Kendrick perspective, the mass of the methylene (CH2) unit is set at exactly 14 Da. The International Union of Pure and Applied Chemistry (IUPAC) mass in daltons can be converted to the mass on the Kendrick scale by division by 1.0011178. The basis in the methylene unit can also be shifted to other functional groups, creating a mass scale linked to that group. The Kendrick mass defect is the difference between the nominal Kendrick mass and the exact Kendrick mass, in parallel to the IUPAC definitions. The value of this shift in perspective advocated by Kendrick is illustrated in the process called a Kendrick mass analysis. In this analysis, the Kendrick mass defect is plotted against the nominal Kendrick mass for ions in the mass spectrum (Figure 3). Successive members of a homologous series (differing in the number of methylene units) are represented by dots (representing ions observed in the mass spectrum) on the horizontal axis. After the composition of one ion is established, the composition of other ions can be established via the position on the plot. The Van Krevelen diagram, described in 1950, also adopted a perspective-shifting approach, but focused on other functional groups (17).
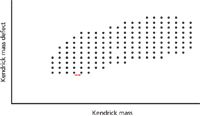
Figure 3: A Kendrick mass analysis plot, with Kendrick mass defect on the y-axis, and Kendrick mass on the x-axis. Successive dots on an x-axis line represent ions observed (or not observed; a binary observation) from related compounds separated by a methylene unit (see red bar). Figure adapted from reference 22.
Kendrick was confronting a daunting world of MS data recorded at a resolving power of 5000. We have advanced to the routine measurement of petroleomics data at 10–15× that resolving power (18) and extending to 10× higher mass, but the need for a meaningful display of the data persists. The Kendrick mass defect is just one of several "mass defects" that can inform our interpretation of mass spectrometric data (19). Increasingly, the methods of informatics give us new tools to approach the mass spectrometric data. The use of Kendrick mass data and Van Krevelen diagrams have been discussed within the larger context of high-precision frequency measurements recorded by many instrumental platforms, and the use of those data to discern complex molecular structures (20,21).
Conclusions
We use eponymous terms in mass spectrometry because they efficiently convey information as well as crystallize and honor the achievements of an individual. Table I presents only a few such eponymous terms; many more may be in limited and specialized use. Whether they enter a more general use ultimately depends on the consensus of the community, which develops over years. Discovering the background of eponymous terms used in mass spectrometry provides a useful perspective of the development of the field.
References
(1) http://www.organic-chemistry.org/namedreactions/.
(2) http://www.monomerchem.com/display4.html.
(3) http://www.chem.ox.ac.uk/vrchemistry/nor/.
(4) http://en.wikipedia.org/wiki/List_of_inventions_named_after_people.
(5) C.F. Brannock, as described in the New York Times of June 9, 2013. See http://www.brannock.com/cgi-bin/start.cgi/brannock/history.html.
(6) J. De Laeter and M. D. Kurz, J. Mass Spectrom. 41, 847–854 (2006).
(7) W. Paul and H. Steinwedel, Zeitschrift für Naturforschung 8A, 448–450 (1953).
(8) R.E. March, J. Mass Spectrom. 32, 351–369 (1997).
(9) G. Stafford, Jr., J. Amer. Soc. Mass Spectrom. 13, 589–596 (2002).
(10) W.M. Brubaker, Adv. Mass Spectrom. 4, 293–299 (1968).
(11) W. Arnold, J. Vac. Sci. Technol. 7, 191–194 (1970).
(12) P.E. Miller and M.B. Denton, Int. J. Mass Spectrom. Ion Proc. 72, 223–238 (1986).
(13) S. Wright, S. O'Prey, R.R.A. Syms, G. Hong, and A.S. Holmes, J. Micromechanical Syst. 19, 325–337 (2010).
(14) K.L. Busch, Spectroscopy 27(1), 14–19 (2012).
(15) K.L. Busch, Spectroscopy 28(3), 14–18 (2013).
(16) E. Kendrick, Anal. Chem. 35, 2146–2154 (1963).
(17) D.W. Van Krevelen, Fuel 29, 269–284 (1950).
(18) C.A. Hughey, C.L. Hendrickson, R.P. Rodgers, A.G. Marshall, and K. Qian, Anal. Chem. 73, 4676–4681 (2001).
(19) L. Sleno, J. Mass Spectrom. 47, 226–236 (2012).
(20) N. Hertkorn, C. Ruecker, M. Meringer, R. Gugisch, M. Frommberger, E.M. Perdue, M. Witt, and P. Schmitt-Koplin, Anal. Bioanal. Chem. 389, 1311–1327 (2007).
(21) T. Grinhut, D. Lansky, A. Gaspar, M. Hertkorn, P. Schmitt-Kopplin, Y. Hadar, and Y. Chen, Rapid Comm. Mass Spectrom. 24, 2831–2837 (2010).
(22) https://en.wikipedia.org/wiki/Kendrick_mass.
Kenneth L. Busch doesn't have anything in mass spectrometry named after him or any pictures for sale on eBay. As a consolation prize, he does have beer, a theme park and gardens, and a company that markets vacuum equipment, all of which carry the name "Busch." None of these have any connection to the author, but the "Busch" neon sign purchased at the brewery gift shop sometimes provides a new perspective. This column is the sole responsibility of the author, who can be reached at wyvernassoc@yahoo.com

Kenneth L. Busch

LIBS Illuminates the Hidden Health Risks of Indoor Welding and Soldering
April 23rd 2025A new dual-spectroscopy approach reveals real-time pollution threats in indoor workspaces. Chinese researchers have pioneered the use of laser-induced breakdown spectroscopy (LIBS) and aerosol mass spectrometry to uncover and monitor harmful heavy metal and dust emissions from soldering and welding in real-time. These complementary tools offer a fast, accurate means to evaluate air quality threats in industrial and indoor environments—where people spend most of their time.
NIR Spectroscopy Explored as Sustainable Approach to Detecting Bovine Mastitis
April 23rd 2025A new study published in Applied Food Research demonstrates that near-infrared spectroscopy (NIRS) can effectively detect subclinical bovine mastitis in milk, offering a fast, non-invasive method to guide targeted antibiotic treatment and support sustainable dairy practices.
Smarter Sensors, Cleaner Earth Using AI and IoT for Pollution Monitoring
April 22nd 2025A global research team has detailed how smart sensors, artificial intelligence (AI), machine learning, and Internet of Things (IoT) technologies are transforming the detection and management of environmental pollutants. Their comprehensive review highlights how spectroscopy and sensor networks are now key tools in real-time pollution tracking.