Field Research and Experiential Learning with Undergraduates: Investigations of Roman Glass Tesserae by Portable X-Ray Fluorescence Spectroscopy
An application of atomic spectroscopy for experiential learning through archaeological investigations is presented.

An application of atomic spectroscopy for experiential learning through archaeological investigations is presented. Undergraduate students participating in an archaeology field school in Italy had the opportunity to utilize portable X-ray fluorescence (XRF) spectrometry to analyze many artifact types including the glass tesserae examined in this study. Approximately 10 different glass tesserae types, identified visually by color, were investigated qualitatively. The elements found agree with previously published studies of Roman glasses. Variations within specific glass colors are identified and discussed.
Although classroom instruction is an essential aspect of most educational programs, experiential learning can have a significant and often longer-lasting impact. These hands-on applications and extensions beyond the classroom — internships, directed research, service learning, and experiences abroad — can lay the foundation for a future career or, at the least, allow students to reflect on how to apply classroom learning in a practical way.
Our experiential learning projects did not start as such; instead they began in 2004 with the seemingly innocuous request to develop an analytical chemistry laboratory experiment for an "Introduction to Archaeology" course taught within the Saint Anselm College Department of Classics. Soon after that work had been completed, a request was made for elemental analyses of samples from an archaeological site. This was followed up by discussions of travels to the site to better understand the context of the samples. Then everything got much more exciting, archaeometrically speaking, when the use of portable spectroscopic instrumentation at the excavation site was proposed for the 2008 dig season.
The Saint Anselm College Field School in Archaeology
Saint Anselm College has offered a summer field archaeology experience for typically 25–35 students in Italy since 2006. The three sites excavated by the team date from the 10th century before the common era (BCE) to the 17th century common era (CE) based on ceramic, numismatic, and literary source evidence. These sites are located in and near the city of Orvieto, Italy. The excavations are directed by David George and Claudio Bizzarri under the auspices of the Soprintendenza per I Beni Archeologici dell'Umbria and the Parco Archeologico e Ambientale dell'Orvietano. Students live together and work together for six to eight weeks, learning the basics of archaeological excavation as well as the historic and archaeological context of the sites and finds. Each season brings about new knowledge of the people that inhabited and visited these sites, and new discoveries including walls, floors, tiles, pottery, water pipes, coins, frescoes, and tesserae.
Many of the tools used for excavating — shovels, pickaxes, buckets, brushes, and trowels — have not changed considerably in the last 200 years. Field archaeology is slow and careful; after all, this is an experiment that cannot be repeated. For Professor Donais, an analytical chemist with early career training at the National Institute of Standards and Technology (NIST), this was an unfamiliar way of thinking. Often, the archaeological questions being asked are of a nature that does not require the ultimate in accuracy and precision. They are important questions nonetheless, and have led us to develop data processing and evaluation procedures appropriate for those questions and our data. Additionally, involvement in this research has provided the unique opportunity for nonchemists to learn about spectroscopy and for chemists to learn about the Etruscans, Romans, pottery types, fresco production, and many other aspects of the ancient world.
Archaeometry at the Field School
To date, our research team has used portable energy-dispersive X-ray fluorescence (EDXRF) spectrometry and portable Raman spectroscopy to explore mortars and cements (1,2), tiles and floors (3), pottery (4), and frescoes (5) at our sites. Instrumental data and the associated field documentation are almost entirely collected by undergraduate students after supervised training at the start of each excavation season. Students can have any academic background to participate in the archaeometric investigations, although most are chemistry, classics, or archaeology majors. Investigations of glass tesserae were started by our research team in 2013. Glass tesserae are plentiful at one of the sites, although unfortunately only rarely intact within mosaic floors or walls. With the significant number of specimens and their range of colors and compositions, glass tesserae present us with a large sample set to explore.
Glass Tesserae
Glass is mostly silica obtained from sand. To lower the melting temperature of the silica and increase the time it takes to solidify, sodium carbonate (soda) or equivalent compounds are added as a fluxing agent; natron, a hydrated sodium carbonate often mixed with other sodium salts, was the sole fluxing agent used by glassmakers from the Roman period. Impurities often found in sand and other purposeful additives contribute to the properties of the glass. Alumina and alkaline Earth oxides help to impart water resistance. Manganese oxide facilitates the removal of gas bubbles, improves the glass's homogeneity during melting, and can act as a decolorant. Calcium antimonate acts as an opacifier (6). And lastly, certain elements impart color to glass depending on their oxidation state and the position the metal occupies in the glass structure. Examples include copper for blue, manganese for purple, iron for browns and yellows, and copper for greens and aquamarines (7,8).
With a considerable amount of glass characterization tied to elemental composition, atomic spectroscopy techniques are often used for analyses of glasses. Research studies of glasses utilizing laser-ablation inductively coupled plasma–mass spectrometry (LA-ICP-MS) (9), laser-induced breakdown spectroscopy (LIBS) (10), X-ray microanalysis (11), and X-ray fluorescence (XRF) (12) have been published recently. The study presented here used EDXRF, a nondestructive and portable technique often used with samples of cultural significance.
Experimental
The glass tesserae were found at the Coriglia, Castel Viscardo archeological excavation site. There were 10 different types as identified visually by color and shown in Figure 1: (top, left to right) aqua, clear blue, light blue, and dark blue; (middle, left to right) red and black; (bottom, left to right) teal, clear green, light green, and dark green. The glasses not indicated as "clear" were opaque. Selected whole tesserae of each type were each labeled with a capital letter (A, B, C, and so on) for differentiation throughout the study.

Figure 1: Glass tesserae.
A Bruker AXS Tracer III-V energy-dispersive portable XRF system with an Rh target X-ray tube excitation source and Si Pin diode detector was used for this work. Data were collected with a 40-keV X-ray tube energy, 8-μA tube current, the Bruker yellow filter, and 180-s analysis times. The XRF spectral data were live time corrected, converted into a text format, and imported into Excel (Microsoft) using a macro. The spreadsheet data were then imported into The Unscrambler (Camo) software for statistical analysis or into ARTAX (Bruker) for peak area determinations. Data were examined qualitatively.

Figure 2: (a) XRF spectra of blue tesserae. (b) Three-dimensional scores plot for blue tesserae.
Results and Discussion
XRF spectra for the 10 tesserae colors are provided in Figures 2, 3, and 4. Spectra were normalized to the Rh signals at about 18–20 kV for ease of viewing multiple spectra within one set of axes. The spectra for the blue tesserae are grouped together in Figure 2, those for the green tesserae are grouped together in Figure 3, and those for the black and red tesserae are grouped together in Figure 4. As noted earlier, glass color is related to metal additives. Spectra for tesserae within color groups are clearly similar.

Figure 3: XRF spectra of green tesserae.
Blue Tesserae
The blue tesserae all had copper present in consistent amounts within each specific color sample set. As previously indicated, copper is a common additive to impart a blue color to glasses (8). Copper was found to be in the highest concentration in the aqua tesserae. The clear blue tesserae had little antimony compared to the other blue glasses and supports the visual observation that an opacifier was not added. Lastly, the tin present in the aqua tesserae is consistent with its green-blue color (13).
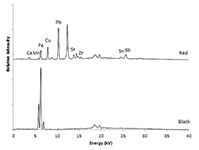
Figure 4: XRF spectra of red and black tesserae.
Lead, also present in all the blue tesserae, showed a wide range of concentrations within each color sample set. This variability is consistent with previous work on Roman glasses (13). Differing lead levels were especially apparent within the dark blue sample set as demonstrated in the bottom two spectra in Figure 2a showing elevated lead in one and little lead in the other. To further explore the elemental differences within the dark blue tesserae, a fully cross-validated principal component analysis (PCA) with equal weighting of the spectral peak areas was performed. The three-dimensional (3D) scores plot is shown in Figure 2b. This model accounted for 99.6% of the observed variance in the first three PCs. The four tesserae in the middle of the scores plot (B, D, J, and H) are clearly grouped together as are those to the right (A, C, I, and E), whereas G (lower left) and F (top middle) are clearly separate from both groups. Upon examination of the spectra and the PCA loadings plot, it is apparent that samples B, D, J, and H all had elevated lead and lower antimony levels compared to samples A, C, I, and E, which had lower lead and elevated antimony. Sample G was unique with higher iron and copper compared to all the others, whereas sample F had significantly higher lead than all the others. Clearly, a more detailed and extensive study of the dark blue tesserae is warranted, especially if the sample set can be increased significantly beyond the 10 samples examined here.
Green Tesserae
All the opaque green tesserae had significant amounts of copper present and were consistent with previously reported work on Roman glasses (10). As noted earlier for the blue tesserae, the lead levels exhibited a wide range of concentrations, with the light green and dark green being more consistently at higher levels. The clear green had manganese present that could have functioned as a decolorant (8) as well as very little antimony, which supports the visual observation that it was not used here as an opacifier.
Red and Black Tesserae
Very few red and black tesserae have been found at the Coriglia site compared to the other glass colors. With only two specimens for each of these colors, no conclusions can be made thus far about elemental consistency across samples. The red glass has significant amounts of lead and some copper and iron. Particulate metallic copper or cuprite (Cu2O) were previously reported to have been used to impart a red color in glasses (8,14) and supports this observation. A reason for the high lead content is not known. The black glass with its high manganese and high iron is similar in composition to glasses reported previously and is likely a very dark purple (15,16).
Conclusion
Atomic spectroscopy, specifically portable XRF, played a valuable role in our initial qualitative studies of Roman glass tesserae. With no sample preparation required, data collection was straight forward and quick. Spectral interpretation required some practice, but was quickly learned by the undergraduate students utilizing this instrumental technique for archaeological sample characterization. Based on these initial findings, additional and more-detailed studies are now planned.
With the successes of this and the other archaeometric studies conducted thus far by our team, our plan is to pull them together with other appropriate topics into a general education science course titled "Chemistry, Art, and Archaeology." It is our hope that we can include portable XRF in this course together with molecular techniques like Raman spectroscopy, and thus provide nonscience majors with hands-on experiences on modern chemical instrumentation while also increasing their exposure to science, technology, engineering, and mathematics (STEM) applications.
Acknowledgments
The authors would like to thank Bruker AXS for the generous loan of the portable X-ray fluorescence spectrometer used for this study. Their continued support of our research and teaching is greatly appreciated.
References
(1) M.K. Donais, B. Duncan, D. George, and C. Bizzarri, X-Ray Spectrom. 39, 146–153 (2010).
(2) M.K. Donais and D. George in Handheld XRF for Art and Archaeology, A. Shugar and J. Maas, Eds. (Leuven University Press, Leuven, Belgium, 2012), pp. 349–377.
(3) M.K. Donais, B. Duncan, S. Wojtas, A. Desmond, and D. George, Appl. Spectrosc. 66, 1005–1012 (2012).
(4) M.K. Donais and D. George, "Spectroscopic Examination of First Pyramidal Hypogeum Found in Etruria and Italy," presnted at SciX 2013 by the Federation of Analytical Chemistry and Spectroscopy Societies, Milwaukee, Wisconsin, 2013.
(5) M.K. Donais, D. George, B. Duncan, S. Wojtas, and A. Daigle, Anal. Methods 3(5), 1061–1071 (2011).
(6) C. Moretti and S. Hreglich in Modern Methods for Analysing Archaeological and Historical Glass, K. Janssens, Ed. (John Wiley & Sons Ltd., West Sussex, United Kingdom, 2013), pp. 23–47.
(7) I. Biron and M.-H. Chopinet in Modern Methods for Analyzing Archaeological and Historical Glass, K. Janssens, Ed. (John Wiley & Sons Ltd., West Sussex, United Kingdom, 2013), pp. 49–65.
(8) A.M. Pollard and C. Heron in Archaeological Chemistry (The Royal Society of Chemistry, London, England, 2008), pp. 144–192.
(9) M. Smirniou and T. Rehren, J. Archaeol. Sci. 40(12), 4731–4743 (2013).
(10) T. Palomar, M. Oujja, M. García-Heras, M.A. Villegas, and M. Castillejo, Spectrochim. Acta, Part B87, 114–120 (2013).
(11) E. Neri and M. Verità, J. Archaeol. Sci. 40(12), 4596–4606 (2013).
(12) B. Dal Bianco and U. Russo, J. Non-Cryst. Solids 358(2), 368–378 (2012).
(13) R. Arletti, S. Conte, M. Vandini, C. Fiori, S. Bracci, M. Bacci, and S. Porcinai, J. Archaeol. Sci. 38(1), 79–88 (2011).
(14) I.C. Freestone in Early Vitreous Materials, M. Bimson and I.C. Freestone, Eds. (British Museum Occasional Paper 56, 1987), pp. 173–191.
(15) S. Cagno, P. Cosyns, A. Izmer, F. Vanhaecke, K. Nys, and K. Janssens, J. Archaeol. Sci. 42, 128–139 (2014).
(16) S. Cagno, P. Cosyns, K. Nys, and K. Janssens in Modern Methods for Analyzing Archaeological and Historical Glass, K. Janssens, Ed. (John Wiley & Sons Ltd., West Sussex, United Kingdom, 2013), pp. 369–385.
Mary Kate Donais received her BS in Chemistry from Bucknell University in 1991 and her PhD in Analytical Chemistry from the University of Massachusetts, Amherst in 1996. Following positions at the National Institute of Standards and Technology and VG Elemental, she joined the faculty at Saint Anselm College in 1999 where she is currently a professor in the Chemistry Department. The current focus of Mary Kate's research is the spectroscopic characterization of archaeological samples. To date, her research group has investigated mortars, cements, floor tiles, frescoes, and glass tesserae utilizing mostly atomic spectroscopy techniques. She has participated in four archaeological excavations in Italy. Mary Kate is actively involved with the Society for Applied Spectroscopy and FACSS.

Mary Kate Donais
Monica Redente graduated from St. Anselm College in May 2014, with a bachelor's degree in chemistry and a mathematics minor. She is planning on pursuing a career in analytical chemistry. Currently, she is working at Dartmouth College as a Research Technician in the Trace Elemental Analysis Lab where she primarily analyzes samples for trace elements via ICP-MS.

Monica Redente
David George is a professor of Classics at Saint Anselm College. He teaches courses in Greek, Latin, and Hebrew as well as the archaeology of Greece and Rome. He has done archaeological work in both Greece and Italy. Currently, he directs a series of archaeological excavations in and around Orvieto, Italy. One can follow these excavations at digumbria.com He has published widely on topics from Greek tragedy to Latin Epic, Biblical Hebrew as well as aspects of Greek and Roman archaeology.

David George

Applications of Micro X-Ray Fluorescence Spectroscopy in Food and Agricultural Products
January 25th 2025In recent years, advances in X-ray optics and detectors have enabled the commercialization of laboratory μXRF spectrometers with spot sizes of ~3 to 30 μm that are suitable for routine imaging of element localization, which was previously only available with scanning electron microscopy (SEM-EDS). This new technique opens a variety of new μXRF applications in the food and agricultural sciences, which have the potential to provide researchers with valuable data that can enhance food safety, improve product consistency, and refine our understanding of the mechanisms of elemental uptake and homeostasis in agricultural crops. This month’s column takes a more detailed look at some of those application areas.
Portable and Wearable Spectrometers in Our Future
December 3rd 2024The following is a summary of selected articles published recently in Spectroscopy on the subject of handheld, portable, and wearable spectrometers representing a variety of analytical techniques and applications. Here we take a closer look at the ever shrinking world of spectroscopy devices and how they are used. As spectrometers progress from bulky lab instruments to compact, portable, and even wearable devices, the future of spectroscopy is transforming dramatically. These advancements enable real-time, on-site analysis across diverse industries, from healthcare to environmental monitoring. This summary article explores cutting-edge developments in miniaturized spectrometers and their expanding range of practical applications.