News
Article
FT-IR Analysis of pH and Xylitol Driven Conformational Changes of Ovalbumin–Amide VI Band Study
Author(s):
Globular protein ovalbumin (OV) contains four secondary structural conformations: α-helix, β-sheet, β-turn, and random coils. The transition between these conformational changes of proteins can be obtained by changes in various chemical and physical processes, including but not limited to heat, pressure, pH, adding cosolvent, and radiation exposure. The conformational changes are being well studied by Fourier transform infrared spectroscopy (FT-IR) spectroscopy, including various spectral regions such as amide A, amide B, and amide I to amide VII. The present study deals with the amide VI band (590–490 cm–1) analysis of OV conformational changes that occurred by varying pHs in the presence of cosolvent xylitol (XY). The obtained result indicates that α-helix to β-sheet transformation favor at pH 2, whereas β-sheet to β-turn transformation favor at pH 12. The neutral pH 7 favors both β-sheet as well as β-turn structures. These results confirmed that the noncovalent interactions contribute a major role in the conformational changes of OV.
Globular proteins play an important role in food, biotechnology, and pharmaceutical applications due to their unique functional properties (1,2). In the globular protein family, the globular protein ovalbumin(OV) has been used as a model protein for the analysis of protein dynamics and properties, and also acts as a surfactant to produce emulsions or foams (3–7). OV is the main part of chicken egg white. Its molecular weight is 45 k Da, and it has a single chain of 385 amino acid residues. It has one disulfide bond and four free sulfhydryl groups and an isoelectric point (pI) of protein is 4.8. The OV is one of the major functional ingredient in the food industry, and its functional property is modulated by various factors such as pH variation, ionic strength, thermal treatment, and adding cosolvents (2,4,8).
As regards cosolvent, sugar is an important ingredient or cosolvent in food to modulate the texture, flavor, and color of protein. At higher levels of consumption, sugar leads to heavier health risks, such as oxidation stress, inflammation cancer, and obesity. Sugars have higher calories than artificial sweeteners, which is the most important reason for the usage of artificial sweetener. Among the artificial sweeteners, xylitol (XY) is one of the high nutritive sweetener of polyhydroxy compound. XY plays a vital role in the food industry as a substitute for sucrose to develop the color and taste of new food products and reduce calories (9,10). In addition, the sugars and polyhydric alcohols contribute a major part in stabilizing biological macromolecules in an aqueous solution (11). Proteins and functional polyhydric alcohols are important food ingredients in many food products, and, therefore, the interaction between them is highly interesting. Recent studies (1,12) have investigated the interaction of XY with globular proteins by ultrasonic, molecular simulation, multispectral techniques, and molecular docking methods. The present study deals with the systematic analysis of the interaction of OV with cosolvent XY in different pHs (2,5,7,9, and 12) by using a Fourier transform infrared (FT-IR) study. The prime significance of FT-IR spectroscopy for the analysis of protein structural characteristics is the applicability to any size of the protein molecule, as well as any physical state of the sample (13). Thin film, solid form, and aqueous solution can be studied in FT-IR analysis (14–16). FT-IR analysis in protein has nine characteristic bands, such as amide A, amide B, and amides I through VII (17). The amide I band (1600–1700 cm−1) represents stretching vibrations, which corresponds to C=O stretching. Among these various bands, amide I is the most applied band by researchers for the characterization of protein secondary structure (18). However, the amide VI band was attempted in few studies for the analysis the globular protein secondary structures (14,19). The amide VI band region is 590–490 cm-1 in out-of-plane bending, which provides rich information about secondary structural details about proteins, hence the present study which is specifically focused on amide VI analysis of pH and XY driven conformational changes of OV. The thorough reviews of the literature show that this type of approach is not yet applied to OV+XY combinations.
Materials and Methods
Chicken egg white-based powdered OV purchased from Sigma Aldrich was used for sample preparation. Phosphate buffers of pH 2, 5, 7, 9, and 12 were prepared from both monobasic and dibasic sodium phosphates (Nice Chemicals) aqueous solutions of 0.2 M. XY solutions of (~1M) prepared in phosphate buffers of pH 2, 5, 7, 9, and 12 were used as a solvent for OV (5 mg/mL). The solution pH was measured by a digital pH meter (Hanna Instruments, model HI 98107). After preparation, the stock solution was kept stored at 20 oC overnight. These solutions were then degassed, and each measurement was made after 20 minutes of thermal equilibrium (30.00 ± 0.01 oC).
Experimental Methods
The FT-IR spectra of protein at various pHs 2, 5, 7, 9, and 12 were recorded in the region 4000–450 cm-1 by a Perkin Elmer Spectrum FT-IR instrument at the Archbishop Casimir Instrumentation Centre (ACIC) at St. Joseph’s College (Autonomous), in Tiruchirappalli, Tamilnadu, India.
Second Derivative Method
With the support of the origin 6.0 program, the second derivatives of all spectra of various pHs 2, 5, 7, 9, and 12 can be obtained. Based on these second derivative spectra, the secondary structure of OV is determined. The protein’s secondary structural patterns can be identified in a more direct method using direct quantitative analysis involving the approximation of an IR band absorbance A(ν) of the spectrum by the Lorentzian function.
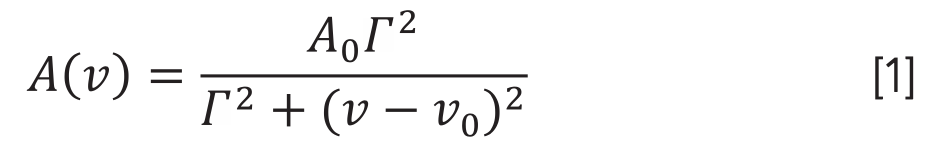
Where ν is the wavenumber (cm-1); A0 is the the maximum absorbance of the band corresponding to wavenumber ν0, and Γ is the half-width at half-height of the original spectrum.
And then in equation 2:
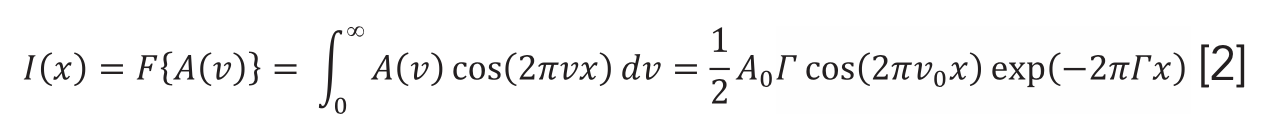
where ν is the frequency (Hz). Between spectrum and its Fourier transform I(x), the following relation holds:
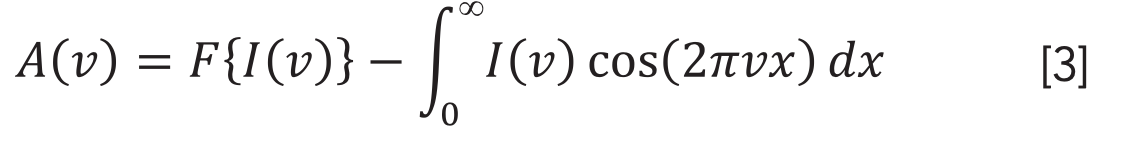
The second order derivative is given by:
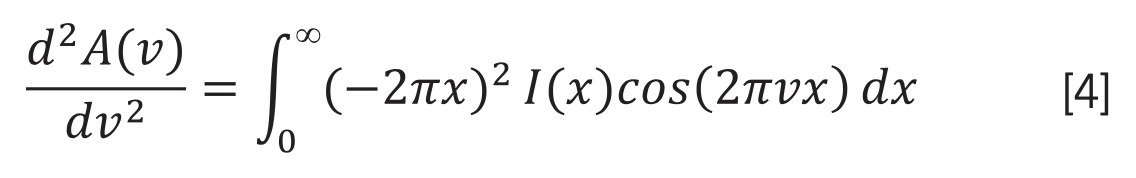
Substituting (2) in (4), the second derivative band spectrum becomes:
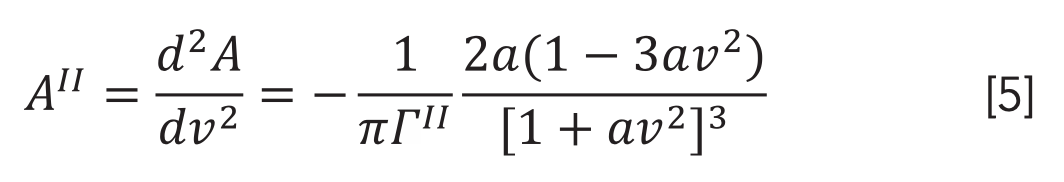
in which:
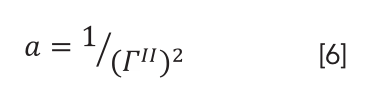
where ΓII is half-width at half-height of the second derivative spectrum. The half-width (ΓII) and the maximum absorbance of the band corresponding to wavenumber ν0(A0II) of the second derivative band spectrum are related to the original line intensity by relations , respectively. By this procedure, the signal-to-noise ratio (S/N) of the spectrum is maximized. Thus, the areas corresponding to the different types of secondary structure are quantitatively evaluated by curve fitting analysis.
Results and Discussion
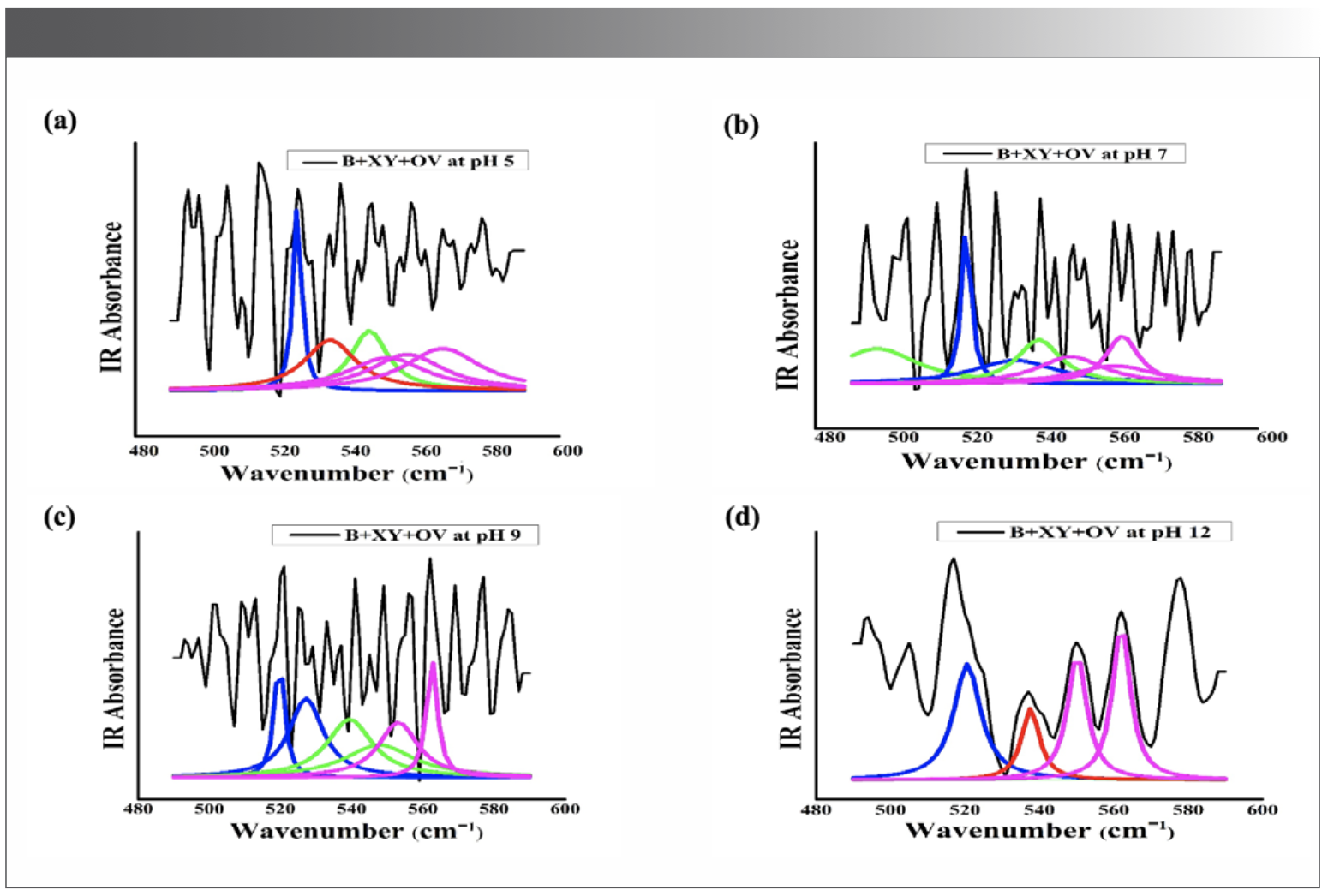
The IR results can be derived from the absorption of energy through vibrating chemical bonds, mostly stretching and bending vibrations. These absorbing frequencies depends on the nature of bands and their vibrations modes. The wavenumber corresponds to a particular frequency of absorption depending on various parameters, such as inter and intramolecular hydrogen bonding, including the peptide bond angle. To resolve the complexity of the FT-IR primary spectrum, the second derivative approach has been applied. In the present study, the second derivative approach has been applied to amide VI band regions. The primary spectra of OV with and without XY in pHs 2, 5, 7, 9, and 12 are shown in Figure 1a.The second derivative FT-IR spectra of the amide VI band of all pHs 2, 5, 7, 9, and 12 are given in Figure 1b and Figures 2a–2d, respectively. In the second derivative spectra, each figure has two patterns—the upper portion indicates the second derivative part, whereas the lower portion indicates the relevant curve fitting part for calculating the area of the assigned band. The quantitative measurement and assignment of peaks in the OV system with and without XY for pHs 2, 5, 7, 9, and 12 are shown in Table III. The characteristic information of infrared bands of peptide linkages and amide VI frequency spectrum of protein secondary structure are summarized in Table I and Table II, respectively. The quantitative assignments of secondary structural contents and their consolidated values are shown in Table III and Table IV, respectively.
FIGURE 1: (a) Primary spectra for OV with and without cosolvent at various pHs; (b) second derivative spectra for pH 2.
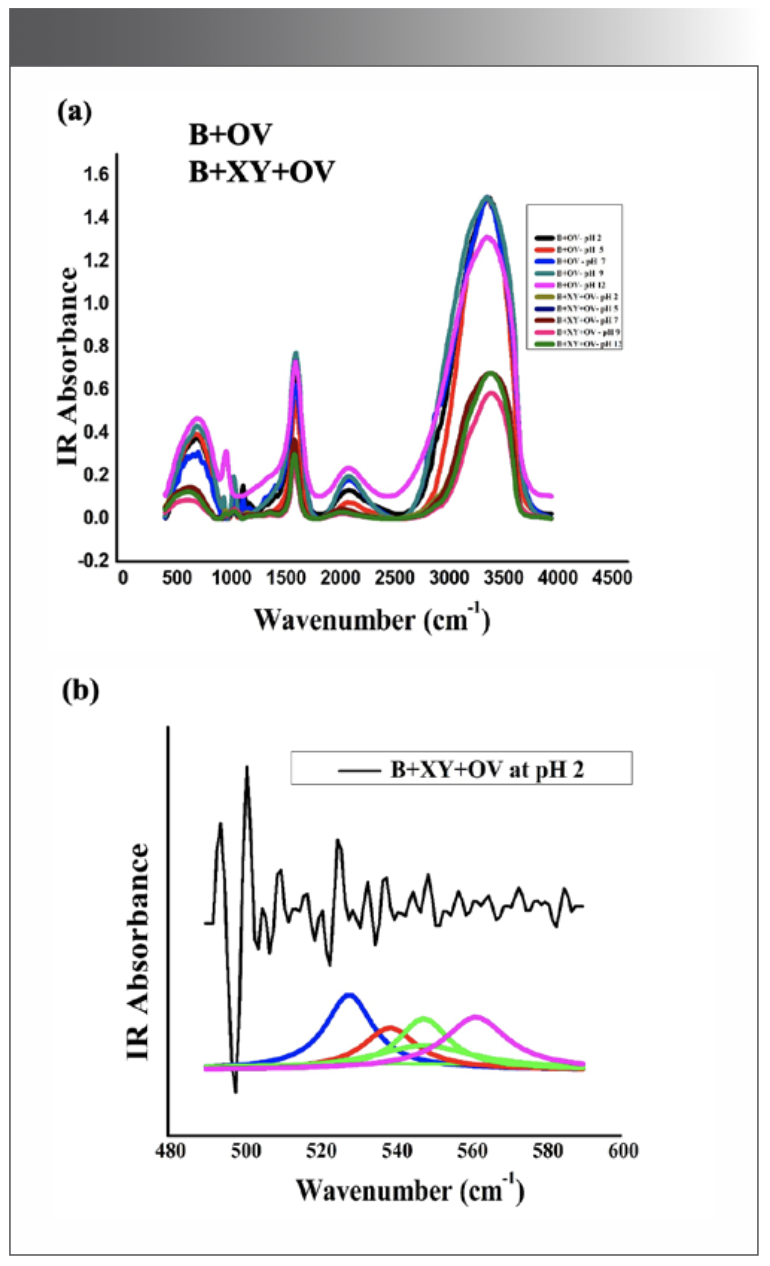
FIGURE 2: (a) Second derivative spectra for pH 5; (b) second derivative spectra for pH 7; (c) second derivative spectra for pH 9; (d) second derivative spectra for pH 12.
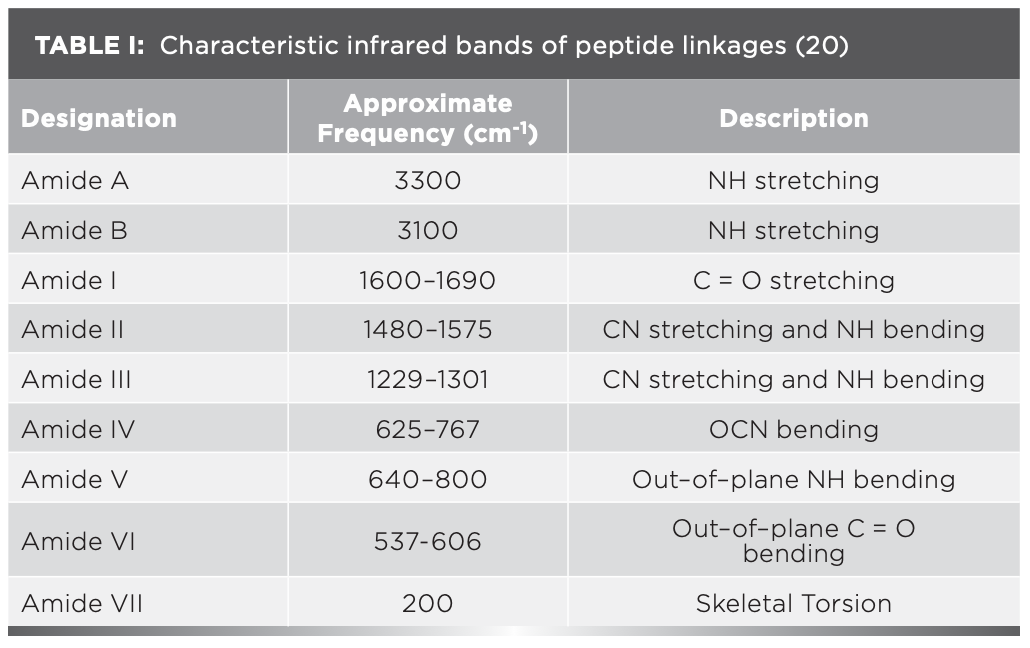
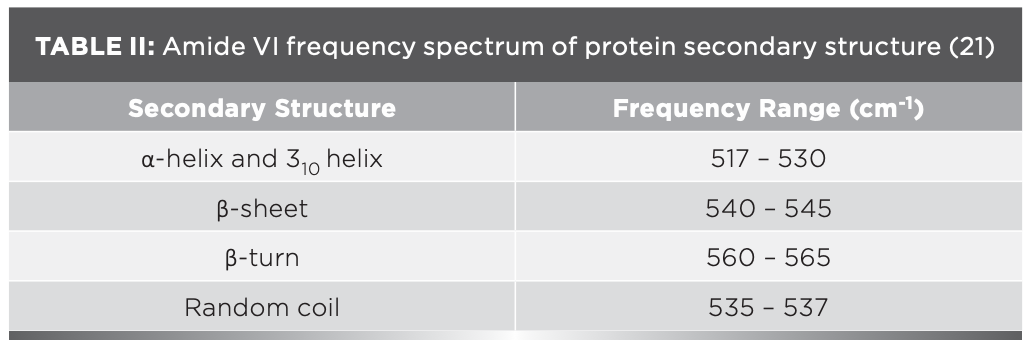
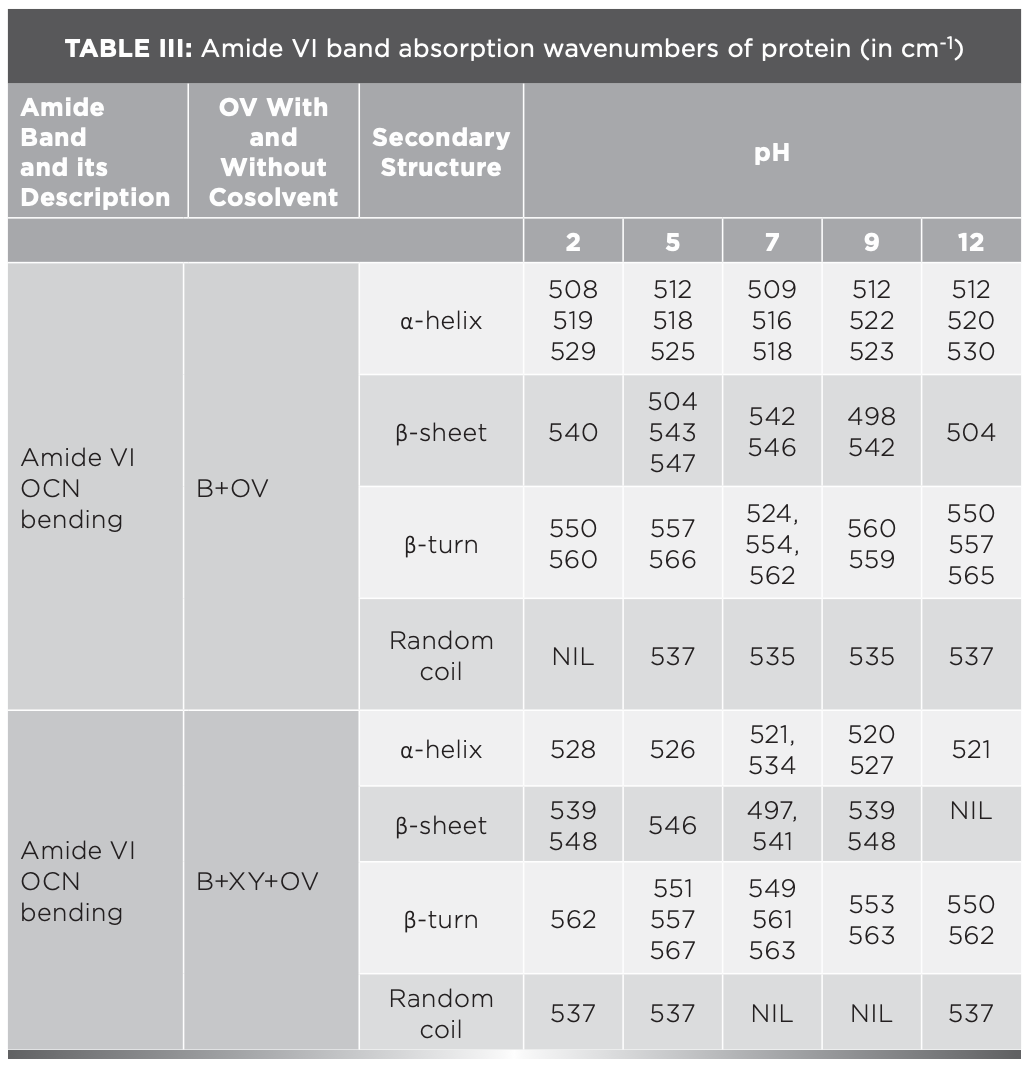
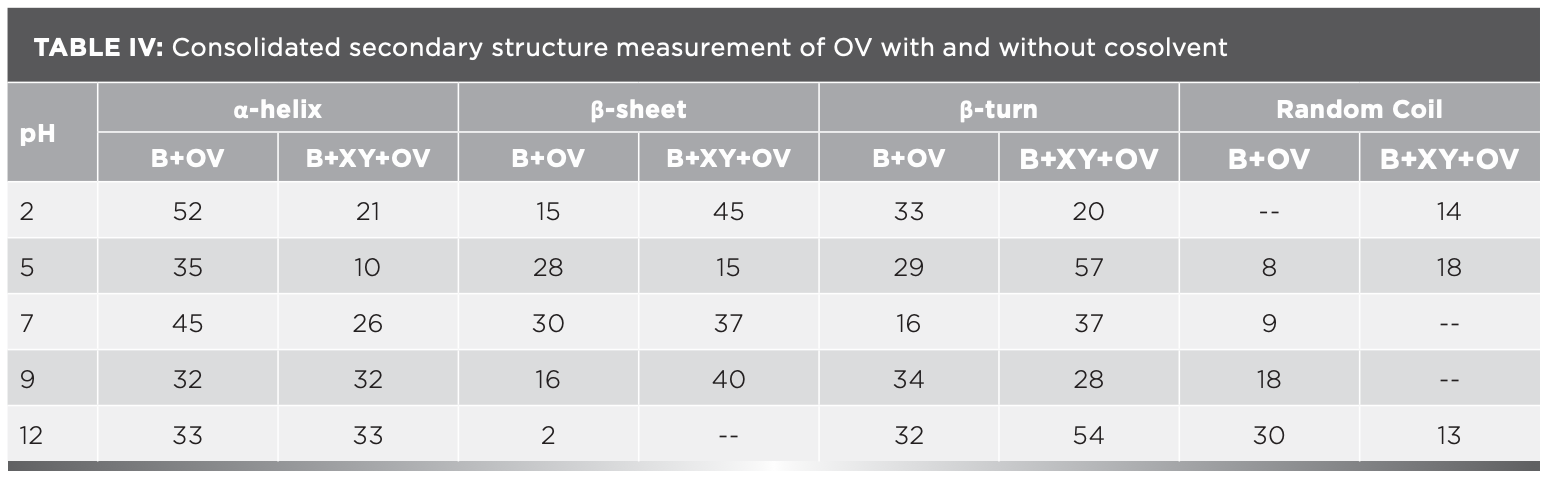
The sample can be identified as simple or complex, based on the number of bands in FT-IR spectra. The major portion of the present system contains proteins; its FT-IR spectrum therefore reveals more than five absorption bands. The peaks noticed at 508 cm-1, 519 cm-1, and 529 cm-1 for OV informing α-helix content is present at pH 2, but the XY addition reduces the helical content, hence 528 cm-1 peak alone was noticed; however, the β-sheet percentage increased. This can be known through the wave numbers 538 cm-1, 539 cm-1, and 548 cm-1, marked by β-sheets.
The increasing trend of β-sheet content due to XY addition is well reflected in the density parameter in ultrasonic study (1), where the researcher reported that the addition of cosolvent fructose with OV tends to decrease the adiabatic compressibility of OV. This situation occurs due to the expulsion of voids in the interior of protein, and leads to an increase in the intermolecular hydrogen bonding mechanism, therefore increasing the β-sheet (20). The same trend occurred in OV, due to the addition of XY.
In the case of pH 5, the α-helix, β-sheet, and β-turn structure are somewhat equal in B+OV. The pH 5 is near the isoelectric point (pI) of a protein, at which the net charge of a protein molecule is zero. It was noticed that a large amount of β-turn at pH 5 compared to other structures (21). Conversion of α-helix and β-sheet into β-turn and random coil structure occurs when XY interacted with whey protein isolate at pH 7.4 in their circular dichroism (CD) studies. The CD spectrum results show that the surface hydrophobicity was reduced, and also increased emulsifying property and increased heat stability of whey protein isolate. It is therefore a tacit assumption that XY addition stabilizes OV through reduced hydrophobicity. A literature survey (22) highlighted that osmolytes stabilize protein through the preferential exclusion process, thereby increasing the solvent viscosity. In an ultrasonic study (1), the authors recorded the higher value of viscosity at pH 5 for the B+XY+OV system. It is clear then that the surface hydrophobicity of OV was reduced due to the preferential exclusion of XY at pH 5.
At pH 7, in the absence of XY, OV was approximately composed of 45% α-helix, 30% of β – sheet, 16% of β-turns, and 9% of random coils (Table IV). In the presence of XY, the α-helix content decreased to 26%, the β-sheet content increased to 37%, the β-turn content increased to 37%, and the random coil content decreased to zero. The reduction in α-helix content occurred probably due to the complex formation between XY and OV (23,24). The same trend was observed by the authors of this paper in their molecular simulation studies (1). It was reported that the oxygen atoms from water are preferentially attracted by the OV acidic pHs, as well as neutral pH. Researchers showed that β-turns influence the stability of the protein’s native state. The turn structure acts as a nucleation point for cooperative formation of neighboring interactions of protein; consequently, it promotes protein stability (25). The larger portion of β-turn state due to XY addition therefore indicates the stabilized state of OV. The larger content of α-helix at pH extremities indicates OV’s molten globule state (MG), a unique state where the major secondary structures of OV are not affected, but the tertiary structure has been lost (26,27). The review of literature was reported that the highly ordered MG state is more favored at acidic pH extremes compared to alkaline pH extremes (27,28). This trend is well in concurrence with the results obtained at pH 2 and pH 12. The aqueous solution of XY has lower dielectric constant than pure water, indicating strong electrostatic interaction compared to water. As OVs are polyampholytes, its charges and hydrophobicity or hydrophilicity are pH dependent. The fluctuations in electrostatic interaction thus are reflected in OV-XY interactions, resulting in conformational changes in OV.
Conclusion
The conformational changes between major secondary structures of OV were found to be due to the variation of pH and inclusion of XY. It was found that α-helix to β-sheet transformation favor at pH 2 whereas β-sheet to β-turn transformation favor at pH 12. The neutral pH 7 favors both β-sheet, as well as β-turn structures. These results confirmed that these noncovalent interactions contribute major role in the conformational changes of OV.
References
(1) Swenthira, K.; Kaique, M.S.; Charles, M.A.; Laudelina, F.A.; Lescano, C.H.; Oliveira, I.P.;Velusamy,V. Molecular Details of Ovalbumin Solvation by an Aqueous Solution of Xylitol in Different pH Environment: Ultrasonic and Molecular Simulation Studies. J.Mol.Liq. 2023, 386 (2), 122477. DOI:10.1016/j.molliq.2023.122477
(2) Velusamy, V.; Palaniappan, L. Effect of pH and Glucose on the Stability of α- Lactalbumin. Food Bio. Phy. 2016, 11 (1), 108–115. DOI 10.1007/s11483-015-9423-2
(3) Miliao, G.L.; Soares, L.S.; Balbino, D.F.; Barbosa, E.A.A.; Bressan, G.C.; Teixeira, A.V.N.C.; Coimbra, J.S.R.; Oliveira, E.B. pH Influence on the Mechanisms of Interaction Between Chitosan and Ovalbumin:A Multi-Spectroscpic Approach. Food Hydrocollo. 2021, 123, 107137. DOI: 10.1016/j.foodhyd.2021.107137
(4) Tan, S.T.; Yu, J.; Lu, L.; Fu, L.Z.; Cai, Z.X.; ZX, X. Diurnal Cycle of IMERG V06 Precipitation. Inter.J.Bio.Mac. 2019, 131, 293–300. DOI: 10.1029/2019GL085395
(5) Tan, J. W.; Joshi, P. Egg Allergy: An Update. Journal of Paediatrics and Child Health 2014, 50 (1), 11–15. DOI: 10.1111/jpc.12408
(6) Livesey, G. Health Potential of Polyols as Sugar Replacers, with Emphasis on Low Glycaemic Properties. Nutr. Res. Rev. 2003, 16 (2), 163–191. DOI: 10·1079/NRR200371
(7) Rajendran, S.; Radha, C.; Prakash, V. Mechanism of Solvent-Induced Thermal Stabilization of α-Amylase From Bacillus Amyloliquefaciens. Int. J. Peptide Protein Res. 1995, 45, 122–128. DOI: 10.1111/j.1399-3011.1995.tb01030.x
(8) Agalya, P.; Velusamy, V.; Pries de Oliveria, I.; Lesanco, C.H.; Caries, A.R.L. Effect of pH and Cosolvent Sucralose on the Solvation Profile of Ovalbumin: Ultrasonic And Molecular Simulation Studies. Food Hydrocollo. 2021, 125, 107386. DOI: 10.1016/j.foodhyd.2021.107386
(9) Chanasattru, E.; Decker, A,; McClements, J. Impact of Cosolvents (Polyols) on Globular Protein Functionality: Ultrasonic Velocity, Density, Surface Tension and Solubility Study. Food Hydrocoll. 2008, 22 (8), 1475–1484. DOI: 10.1016/j.foodhyd.2007.09.007
(10) Torres, M.D.; Raymundo, A.; Sousa, I. Effect of Sucrose, Stevia And Xylitol on Rheological Properties of Gels From Blends of Chestnut and Rice Flours. Polymers 2013, 98 (1), 249–256. DOI:10.1016/j.carbpol.2013.06.018
(11) Chen, Y.; Hu, J.; Yi, X.; Ding, B.; Sun, W.; Yan, F. F. Interactions and Emulsifying Properties of Ovalbumin With Tannic Acid. Food Sci. and Techn. 2018, 95, 282–288. DOI: 10.1016/j.lwt.2018.04.088
(12) Kong, F.; Kang, S.; Tian, J.; Li, M.; Liang, X.; Yang, M.; Yue, X. Interaction of Xylitol With Whey Proteins: Multi-Spectroscopic Techniques and Docking Studies. Food Chem. 2020, 326 (1), 126804. DOI: 10.1016/j.foodchem.2020.126804
(13) Zeeshan, F.; Tabbassum, M.; Jorgensen, L.; Medlicott, A. Attenuated Total Reflection Fourier Transform Infrared (Atr Ft-Ir) Spectroscopy As An Analytical Method to Investigate The Secondary Structure of a Model Protein Embedded in Solid Lipid Matrices. Appl. Spectr. 2018, 72, 268–279. DOI: 10.1177/0003702817739908
(14) Usoltsev, D.; Sitnikova, V.; Kajava, A.; Uspenskaya, M. Systematic FT-IR Spectroscopy Study of The Secondary Structure Changes in Human Serum Albumin Under Various Denaturation Conditions. Biomolecules 2019, 9 (8), 359. DOI: 10.3390/biom9080359
(15) Chinnaiah, K.; Kannan, K.; Krishnamoorthi, R.; Palko, N.; Gurushankar, K. Nanostructured Ag/NiO Composites for Supercapacitor and Antibacterial Applications, and In-Silico Theoretical Investigation. J. Phys. Chem. Solids 2024, 184, 11173. DOI: 10.1016/j.jpcs.2023.111730
(16) Chinnaiah, K.; Kannan, K.; Krishnamoorthy, R.; Gurushankar, K. Datura metel L. Leaf Extract Mediated Sodium Alginate Polymer Membrane for Supercapacitor and Food Packaging Applications. Int. J. Biol. Macromol. 2023, 242, 125112. DOI: 10.1016/j.ijbiomac.2023.125112
(17) Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. DOI:10.1016/j.bbabio.2007.06.004
(18) Agalya, P.; Velusamy, V. Impact of pH and Sucralose on the Non-Covalent Interaction of Ovalbumin: FT-IR Analysis. Spectroscopy 2023, 38 (s8), 23–28. DOI: 10.56530/spectroscopy.tp5468m7
(19) Agalya,P.; Velusamy,V. FT-IR Analysis of Conformational Changes in The Secondary Structure of Ovalbumin: Effect of pH and Cosolvent Sugar-Free Natura. Sci. and Tech. J. 2020, 8 (1), 2321–3388. DOI: 10.22232/stj.2020.08.01.10
(20) Khoury, Y.E.; Hielscher, R.; Voicescu, M.; Gross, J.; Hellwig, P. On The Specificity of the Amide VI Band for The Secondary Structure of Proteins. Vib. Spec. 2011, 55, 258–266. DOI: 10.1016/j.vibspec.2010.12.001
(21) Kong, J.; Shaoning, Y. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39 (8), 549–559. DOI: 10.1111/i.1745-7270.2007.00320.x
(22) Kavitha, K.; Palaniappan., L. Response of Ovalbumin to Fructose Addition and pH Variations - Ultrasonic and FTIR Study. Agri, Food and Environ. Res. 2021, 9 (2), 263–281. DOI: 10.7770/safer-V0N0-art2231
(23) Oliveira, I.P.; Martinez, L. Molecular Basis For Competitive Solvation of The Burkholderia Cepacia Lipase by Sorbitol and Urea. Phy. Chem. Chem. Phy. 2016, 18 (31), 21797–21808. DOI: 10.1039/C6CP01789D
(24) Wei, J.; Xu, D.; Yang, J.; Zhang, X.; Mu, T.; Wang, Q. Analysis of The Interaction Mechanism of Anthocyanins (Aronia Melanocarpa Elliot) With β-Casein, Food Hydrocoll. 2018, 84, 276–281. DOI: 10.1016/j.foodhyd.2018.06.011
(25) Marie, A.; Marcelino, C.; Gierasch, L. Roles of β-Turns in Protein Folding: From Peptide Models to Protein Engineering, Biopoly. 2008, 89 (5), 380–391. DOI:10.1002/bip.20960
(26) Kong, F.; An, Y.; Jiang, L.; Tian, J.; Yang, M.; Li, M.; Zhang, Z.; Guan, B.; Zheng, Y.; Yue, X. Spectroscopic and Docking Studies of the Interaction Mechanisms of Xylitol with α-Casein and κ-Casein. Colloids Surf., B 2021, 206, 111930. DOI: 10.1016/j.colsurfb.2021.111930
(27) Koseki, T.; Kitabatake, N.; Doi, E. Freezing Denaturation of Ovalbumin at Acidic pH, J. Biochem. 1990, 107 (3), 389–394. DOI: 10.1093/oxfordjournals.jbchem.a123055
(28) Tatsumi, E.; Hirose. M. Highly Ordered Molten Globule-Like State of Ovalbumin at Acidic pH: Native-Like Fragmentation by Protease and Selective Modification of Cys367 With Dithiodipyridine. J. Bio Chem. 1997, 122 (2), 300–308. DOI: 10.1093/oxfordjournals.jbchem.a021753
Swenthira Kandasamy is a PhD Scholar in Physics at the Arignar Anna Govt. Arts College, in Namakkal, Tamilnadu, India. Agalya Palanisamy is an Assistant Professor in the Department of Physics at Selvam College of Technology (Autonomous), in Namakkal, Tamilnadu, India. Velusamy Veerappan is an Assistant Professor in the PG & Research Department of Physics at Arignar Anna Govt Arts College, in Namakkal, Tamilnadu, India. Direct correspondence to: veluphysics@yahoo.com●
Newsletter
Get essential updates on the latest spectroscopy technologies, regulatory standards, and best practices—subscribe today to Spectroscopy.




