Handheld Laser-Induced Breakdown Spectroscopy (hLIBS): A Valuable Tool for Terrestrial and Extraterrestrial Recognition of Meteorites in the Field
The role and performance of handheld laser-induced breakdown spectroscopy (hLIBS) devices is discussed in the context of meteorite research exploration while targeting specific questions of geologic interest in the field. The fast data acquisition and minimal sample preparation features of these devices make them very attractive as candidate tools for future astronauts in geochemical mapping, resource prospecting, sample selection, and hazard identification. Although hLIBS instruments have recently become commercially available, they have not yet been widely applied to meteoritical field campaign. This article reviews how the very promising advanced analytical tool hLIBS may be helpful to classify and discriminate meteorites in the field.
The mineralogy, chemistry, and isotopic composition of meteorites can provide unique information on the materials that constitute the solar system and allow deciphering its cosmic history because the composition of many belt asteroids have never changed drastically. The composition of these asteroids has not changed because of gravitational high temperatures and pressures as larger bodies do. Meteorites do not often differ much from terrestrial rocks, a similarity that often compromises their recognition and preservation, leading to their easy disappearance in the landscape should they not be seen to fall and then promptly collected. In particular, iron meteorites can be easily confused with man-made iron objects simulating typical features of a true meteorite. After a careful analysis, a specimen visually thought or suspected to be an iron meteorite may be revealed to be a pseudometeorite, or meteor-wrong. These materials are attracted by a magnet and include terrestrial iron oxides such as magnetite along with many different types of man-made iron byproducts, such as slags and castoff iron with very rough, burned and melted glassy (vitreous) surfaces. There may occasionally be the presence of vesicles (gas bubbles) because corrosion occurred over time.
Although meteorites sometimes appear different from ordinary terrestrial rocks lying around them, their preliminary identification in the field needs a practiced eye. In particular, many meteorites do not contain the common earth mineral quartz, and generally do not present vesicles. Most meteorites, especially iron meteorites, are much heavier in the hand than an ordinary earth rock, and contain a significant amount of iron (Fe) and nickel (Ni). Thus, the first step to identify them is whether they stick to a magnet. However, many terrestrial rocks are also attracted by a magnet, so this test cannot be considered definitive, but only represents a preliminary proof needing of further analysis. Visual and magnetic methods are not exhaustive for a verifiable identification that requires an adequate laboratory analysis to ascertain the extraterrestrial origin of suspected, especially iron, meteorites. In particular, Ni is an element rare in terrestrial rocks, but is present very often in meteorites. Thus, if visual inspection and the magnet test are positive, the presence and content of Ni should be verified, together with that of some other diagnostic trace elements such as gallium (Ga), germanium (Ge), and iridium (Ir).
Analytical Instrumentation for Field Analysis
The availability of analytical instrumentation for routine chemical analysis in the field has been one of the most long-standing needs of the scientific community. The requirements of such a field analyzer include: (i) lightweight and portability, to allow use by one individual; (ii) ruggedized and robust structure for use in a variety of environments; (iii) simplicity of use; and (iv) capability of identifying a large number of elements over a short acquisition time. Many different analytical techniques have been tested in developing a portable instrumentation appropriate for chemical analysis in the field, including mass spectrometry, ion mobility spectrometry, gas chromatography, nuclear magnetic resonance, electron spin resonance, millimeter wave, and terahertz spectrometry, as well as many different forms of optical spectroscopy, such as X-ray fluorescence (XRF), Raman, near-infrared, mid-infrared, ultraviolet–visible, and laser-induced breakdown spectroscopy (LIBS). In particular, XRF and LIBS appear to meet the criteria for performing elemental analysis in the field under ambient environmental conditions.
Instrumentation, Analytical Procedures, and Features of LIBS
The main components of a LIBS instrument are: (a) a laser, the most used being the short-pulsed Q-switched laser that releases a huge amount of energy in a short period, which is used to ablate the sample; (b) a time control system, allowing for the precise control of the beginning of laser pulse, the number of pulses, the interval between pulses, the beginning of spectrum capture, and the acquisition time; (c) a set of mirrors and lenses focusing the laser light onto the sample and collecting the light emitted from the plasma; and (d) a spectrometer–detector system for collecting and spectral discriminating of plasma light emissions that contain all the information on the sample composition and physical conditions of the plasma.
In a LIBS experiment, the high-energy laser pulses are focused on the surface of the sample that is placed in air or an inert gas at ambient or controlled pressure, so to ablate a small portion of it, which is then vaporized, generating the plasma that contains thermally excited atoms, ions, and molecules of the analyte. As the plasma cools down, the excited species decay to lower energy levels emitting electromagnetic radiations typical of each of them, which are collected by a spectrometer that resolves the specific emissions. The acquired signals are then processed, visualized, and analyzed using a dedicated software. An optically thin plasma in the condition of local thermodynamic equilibrium (LTE) is expected to yield a LIBS spectrum whose pattern corresponds to the elemental composition of the sample (1).
Quantitative elemental analysis by LIBS is based on the proportionality between the spectral emission line intensity of the element and its concentration, and can be performed by constructing a classical calibration curve that relates the measured elemental line intensity to the elemental concentration of standards of known composition, or by the calibration free (CF) approach that avoids the use of calibration standards and can address sample matrix issues (2). Furthermore, multivariate statistical methods are used, which can process all spectral information contained in a LIBS spectrum and allow relating them to the composition of the sample, and identify and discriminate different materials (3).
With respect to various destructive and expensive analytical techniques, LIBS features significant advantages, including sensitivity to light elements, versatility, minimal destructivity, rapidity, relatively low operating costs, and the possibility of in-field operations (4). Furthermore, several handheld (h) LIBS instruments, which consist of compact, lightweight, self-contained units that can be used comfortably while being held in the hand of an operator, have been developed and commercialized (4). Recently, hLIBS instruments were used for the rapid in situ measurement of most major and trace elements in a variety of rocks including meteorites, which cannot be achieved by using other field portable instruments.
A Few Representative Examples of hLIBS Application
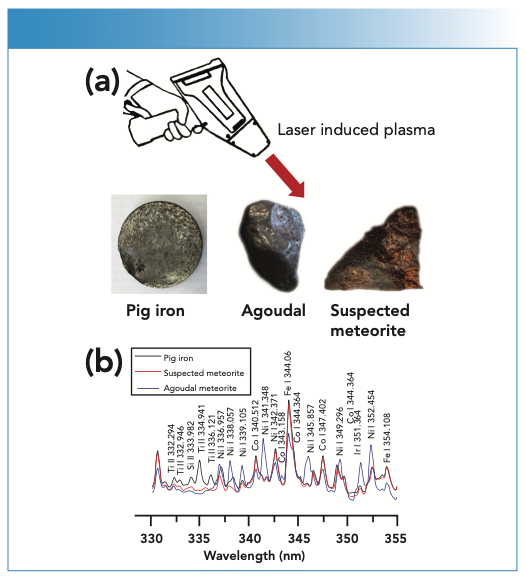
In the last decade, LIBS has shown to be a very promising technique for meteorite analysis and identification (5–7). In two companion works (8,9), a commercial hLIBS instrument was used for the first time to achieve a preliminary chemical identification and classification of meteorites. The objective of one study (8) was to discriminate between a classified and certified meteorite named Agoudal, a suspect meteorite fragment termed meteor-wrong and a pig iron product (Figure 1a) on the basis of their major and trace element composition. A calibration-free (CF)-LIBS approach was used to quantify the main elements Fe, Ni, and Co in the iron meteorite and Si, Fe, Mn, and Ti in the other two samples (Figure 1b). Although the quantitative data obtained were only partially satisfactory, requiring further improvements of hLIBS analyzer components to increase analytical sensitivity, the technique was able to recognize and discriminate between a true meteorite specimen and mislabeled or unlabeled dubious meteorite samples, which allowed a decision to be made about whether to undertake further laboratory analysis or not.
FIGURE 1: (a) Handheld instrument analyzing Agoudal, pig iron, and a suspected meteorite; (b) LIBS spectra acquired on Agoudal meteorite, pig iron, and a suspected meteorite in the spectal range 330–355 nm. Y-axis label is LIBS emission intensity (a.u.).
The same hLIBS instrument was used in a companion effort (9) to discriminate between iron, stony-iron, and stone meteorites and meteor-wrongs (Figure 2). In this work, an innovative strategy was developed allowed the identification and classification of meteorites according to their nature and origin. This novel approach was based on the integration of results of automated feature selection from broadband LIBS spectra and supervised learning by a novel fuzzy-rule-based inference algorithm that could be easily interpreted by human users. The algorithm developed was able to assign correctly 25 out of 26 unclassified meteorite samples to their class with a high total accuracy (96.15%), and identify the most relevant intragroup variations, while only 18 of the samples were properly recognized by partial least square–discriminant analysis (PLS-DA). These results confirm that the classification of LIBS spectra of meteorites is a nonlinear process, and that a high accuracy cannot be achieved using prevalent traditional models, such as principal component analysis (PCA) and PLS-DA, which can only evaluate linear combinations of input variables. Meteorite identification, especially for chemically similar samples, is a challenging decision-making problem that requires a compromise among human experience, visual evidence, and efficient analytical data processing that can be addressed by a fuzzy logic approach. Thus, this approach based on the identification of elements using the six wavelengths selected is expected to be extended to subsequent meteorite investigations, and suggests that such a methodology may also be suitable for other types of chemometric studies.
FIGURE 2: Images of laser beam craters (red circles) obtained by LIBS on (a) a fragment of the whole meteorite chondrite L3, and (b) a suspected meteorite named “meteor-wrong.” (c) LIBS spectra of various meteorites in the spectral range 200–350 nm. Y-axis label is LIBS emission intensity (a.u.).
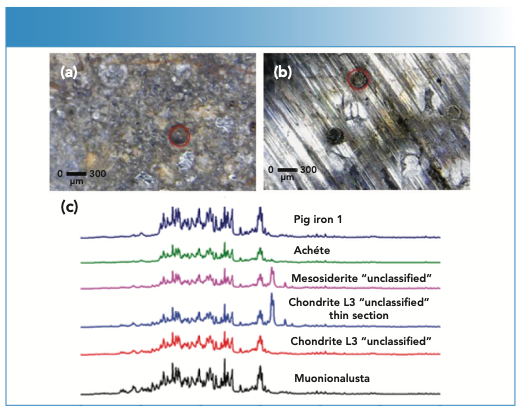
The examples described above confirm that hLIBS is particularly promising for performing minimally destructive (Figure 2 a,b,c) in-situ measurements aiming at a preliminary identification, discrimination and classification of meteorites, and recognition of mislabeled or unlabeled and dubious meteorite specimens in the field, museums, and private collections, as well as deciding about any further laboratory analysis. More widespread applications for real-time analysis in the field are expected to be possible if hLIBS analyzers are used in combination with an on-board global positioning system (GPS) and wireless data transmission devices. Furthermore, it would also be helpful to develop robust algorithms that allow hLIBS data to provide reliable chemical fingerprints for a variety of meteorites, as well as construct spectral libraries to permit sample discrimination and provenance determination in real time.
Future Perspectives
A promising relevant future application of LIBS is expected in the study and analysis of the chondrite class of meteorites, as they are known to preserve the chemistry of the earliest solar system and, to some extent, the pre-solar molecular cloud from which it originated. In particular, carbonaceous chondrites consist in a primitive subgroup of stony meteorites featuring an elemental composition very similar to that of the sun and the overall universe. These meteorites are aggregate rocks, meaning they consist mainly of a matrix of hydrous and anhydrous silicates packed together, which do not show any sign of metamorphism or alteration by high heating. The most intriguing feature of these chondrites is their abundance in carbon (about 1.5–4%), which is mostly present in the organic form. Thus, the investigation of carbon forms and distribution in chondrites can contribute better than most other elements to understanding the early stages of solar system formation, due to the sensitivity of this element to chemical and physical conditions that prevailed in that situation. Recently, an important increase was noticed in the collection of carbonaceous chondrites and achondrites in Africa, although these types of meteorites are very difficult to spot on the ground.
The detection and quantification of free carbon in meteorites have been commonly performed by either destructive techniques, including total organic carbon (TOC) and inductively coupled plasma–mass spectrometry (ICP-MS), or by techniques implying sample preparation, such as scanning electron microscopy (SEM), which uses Au to coat the sample surface, whereas vibrational spectroscopies such as infrared (IR) and Raman are generally used to analyze carbon compounds. Unlike the above techniques, LIBS is able to detect both elemental and combined carbon, even at ppm levels on the whole fragment or its petrographic thin sections, and reveal differences in the spectral emission line of C in different meteorites also in comparison to a meteor-wrong sample (10). This application may be considered a great advance in the context of human space exploration, where hLIBS instruments have shown to be very promising candidate tools in planetary geochemical mapping, resource prospecting, sample selection, and hazard identification.
References
(1) D.A. Cremers and L.J. Radziemski, Handbook of Laser-Induced Breakdown Spectroscopy (John Wiley & Sons, Chichester, United Kingdom, 2nd ed., 2013).
(2) A. Ciucci, M. Corsi, V. Palleschi, S. Rastelli, A. Salvetti, and E. Tognoni, Appl. Spectrosc. 53, 960–964 (1999).
(3) J.L. Gottfried, in Handbook of Laser‐Induced Breakdown Spectroscopy, D.A. Cremers and L.J. Radziemski, Eds., (Wiley, Chichester, United Kingdom, 2nd ed., 2013), pp. 223–255.
(4) G.S. Senesi, R.S. Harmon, and R.R. Hark, Spectrochim. Acta Part B 175, 106013 (2021). https://doi.org/10.1016/j.sab.2020.106013.
(5) G.S. Senesi, Earth-Sci. Rev. 139, 231–267 (2014). https://doi.org/10.1016/j.earscirev.2014.09.008.
(6) G.S. Senesi and F. Capitelli, in Hypersonic Meteoroid Entry Physics, G. Colonna, M. Capitelli, and A. Larucchiuta, Eds., (IOP Series in Plasma Physics, Bristol, United Kingdom, 2019), pp. 1–39.
(7) R.S. Harmon and G.S. Senesi, Appl. Geochem. 128, 104929 (2021). https://doi.org/10.1016/j.apgeochem.2021.104929.
(8) G.S. Senesi, P. Manzari, G. Tempesta, G. Agrosì, A.A. Touchnt, A. Ibhi, and O. De Pascale, Geostand. Geoanal. Res. 42, 607–614 (2018). https://doi.org/10.1111/ggr.12220.
(9) G.S. Senesi, P. Manzari, A. Consiglio, and O. De Pascale, J. Anal. At. Spectrom. 33, 1664–1675 (2018). https://doi.org/10.1039/C8JA00224J.
(10) O. De Pascale, P. Manzari, B. Marangoni, G. Nicolodelli, and G.S. Senesi, Reports of the National Academy of Sciences: Memoirs of Physical and Natural Sciences 138, 25–29 (2020).
About the Author
Giorgio S. Senesi is a researcher at the Institute for Plasma Science and Technology, in Bari, Italy, which is part of the National Research Council (CNR) of Italy. Direct correspondence to: giorgio.senesi@cnr.it

Laser Ablation Molecular Isotopic Spectrometry: A New Dimension of LIBS
July 5th 2012Part of a new podcast series presented in collaboration with the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), in connection with SciX 2012 — the Great Scientific Exchange, the North American conference (39th Annual) of FACSS.