Handheld X-ray Diffraction for Remote, Field-Based Applications
Spectroscopy
This installment describes the development of two novel X-ray diffraction (XRD) techniques that enable the rapid analysis of samples using handheld instrumentation for remote applications. Both techniques can be applied to unprepared samples in the field, which is a highly favorable characteristic in many applications since the time required for laboratory-based sample preparation is avoided.
This installment describes the development of two novel X-ray diffraction (XRD) techniques that enable the rapid analysis of samples using handheld instrumentation for remote applications. Both techniques can be applied to unprepared samples in the field, which is a highly favorable characteristic in many applications since the time required for laboratory-based sample preparation is avoided. The first development is based on back-reflection energy-dispersive XRD, which offers the benefit of being insensitive to sample morphology and therefore opens the possibility of analyzing some sample matrices with no sample preparation at all. The second area of research focuses on using the technology to enhance the XRD signal from a specific mineral or phase within a mixture specifically for metallurgical applications.
This column installment describes the development of two novel X-ray diffraction (XRD) techniques that show exciting potential in handheld and portable applications. Although the ways in which these two techniques work are distinct, they are linked through their applicability in compact instrument formats with similar application areas in mining and metallurgy. The X-ray technologies required for implementation of both techniques are low cost and are essentially the same as those used in existing handheld X-ray fluorescence (XRF) instrumentation, representing a relatively low-risk path to the development of a commercial product.
Back-Reflection Energy-Dispersive XRD
The first of these methods is based on the application of energy-dispersive XRD (EDXRD) in a back-reflection geometry (1,2). This combination yields a property that is essentially unique in the field of XRD--almost complete insensitivity to sample morphology. An idealized configuration for this technique is illustrated in Figure 1, in which the X-ray beam from a tube source passes through an annular detector and is incident on a sample.
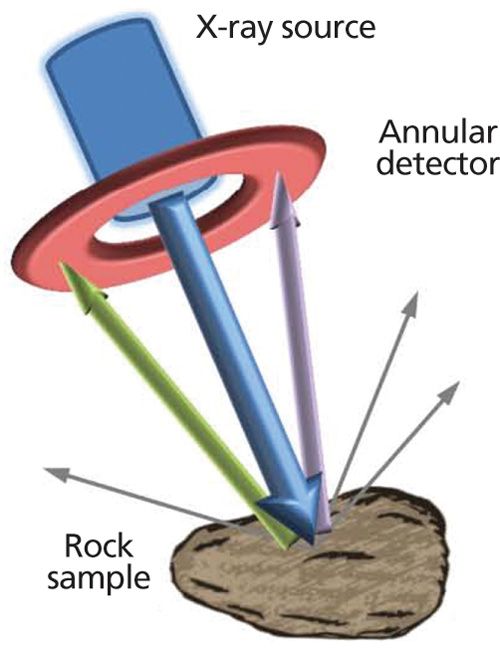
This configuration maximizes the detected diffraction signal from the sample while conforming to the angular constraints of the back-reflection method. The insensitivity to sample morphology arises because, for an X-ray photon diffracted exactly through 180° for example, the distance of the interaction point on the sample relative to the instrument becomes immaterial. It can readily be shown that, in practice, constraining the scattering angle to 2θ > ~160° retains this valuable property (1).
A lack of sensitivity to the shape of the sample opens up the opportunity to analyze samples with no preparation at all, and this is the primary advantage of the back-reflection technique. In addition to the convenience of avoiding the crushing and grinding of samples, and the equipment needed to accomplish this procedure, a considerable time-saving is achieved. In its most basic form, back-reflection EDXRD can be implemented using the same X-ray technologies found in handheld XRF instrumentation, a mature technology with global annual sales of several thousand units into a wide range of end-user applications (3). Furthermore, EDXRD tends to be much faster than the angle-dispersive XRD methods used in the majority of laboratory-based instruments, and the back-reflection geometry is ideal for handheld-based applications. All of these factors come together to favor the commercialization of a handheld device, which is a relatively minor evolution of an existing, highly successful instrument (4).
The primary application envisaged for a handheld XRD instrument of this type is in mining and related areas, with iron ore mining likely to be the single largest commercial application. A handheld XRD device based on back-reflection EDXRD will not be able to compete with laboratory diffractometers in terms of data quality and accuracy, but nevertheless there are major opportunities to add value with rapid XRD measurements in the field. For example, identification of the dominant iron oxide species in an iron ore sample, such as hematite (Fe2O3) or magnetite (Fe3O4), will allow improved XRF accuracy of the total iron content of the sample since the form of the oxide will no longer need to be assumed and the consequent potential errors can be avoided. In addition, the improved accuracy will feed directly through to the XRF quantification of all other elements in the sample. A working prototype instrument has recently been developed by modification of a handheld XRF device. An image of the prototype is shown in Figure 2 and it features a measurement head that has been revised to achieve the required back-reflection geometry.
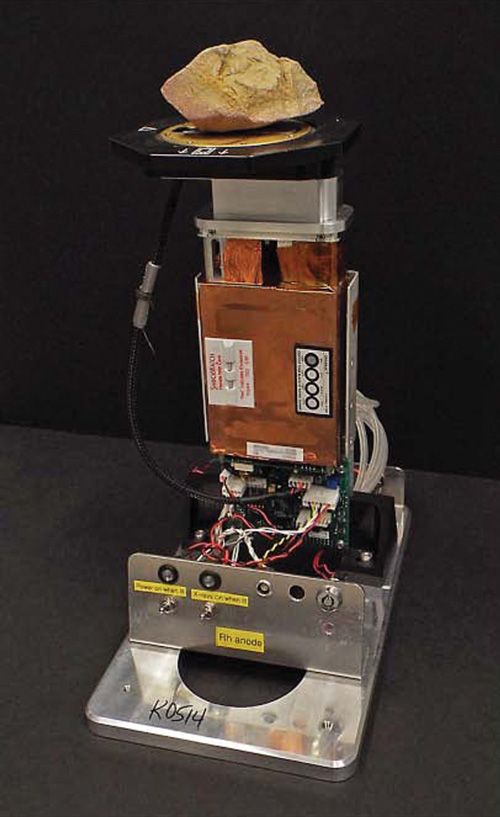
Figure 3 shows the recorded spectrum for a rock sample known to be predominantly hematite, together with a model fit of the diffraction spectrum of hematite.
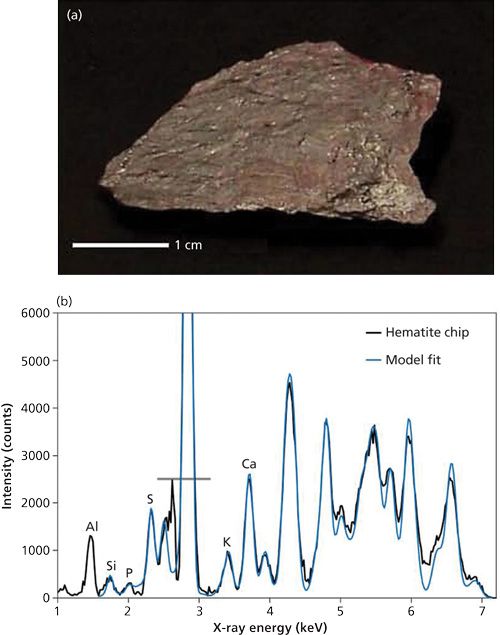
The model separately includes minor amounts of the elements that show fluorescence peaks (labeled on the plot) to achieve a good overall fit. It is important to note that the Rh X-ray tube source emits strong Rh-L lines approximately in the range 2.5-3.1 keV, which scatter from the sample as well as part of the measurement head, and give a signal that dominates the spectrum in this energy range. By recording a spectrum with no sample, the background has been subtracted but the peak intensities in this range are not reliable and have been excluded from the fitting process. The strong off-scale peak in Figure 3 is because of the coincidence of a Rh-Lβ line with a hematite diffraction peak. Overall, the fit to the data is extremely good and demonstrates the potential of the back-reflection technique in handheld XRD analysis of samples of this type. For the results shown here, the measurement head was evacuated, but this would not necessarily be required for a purpose-designed instrument.
Recent laboratory testing with the prototype instrument shows that, in many cases, secondary minerals in iron ore samples can be identified and approximately quantified. For active mining operations, such measurements will enable rapid delineation of ore boundaries and real-time assays at the mine face. The assessment of ore grades will allow more efficient management of blasting, excavation, and haulage operations. The efficiency of the on-site enrichment process can be monitored via the analysis of raw materials, concentrates, and tailings. All of these advantages serve to improve the overall mine productivity while reducing costs relative to sending samples off-site for laboratory analysis. It is anticipated that the same advantages would be realized for other metallic ore mining operations.
Characterization of Limestones
Another very promising application area is the assessment of the quality of dolomitic limestones. Limestone consists mainly of calcite (CaCO3) and is commonly added to the blast furnace in the production of iron to combine with and remove the impurities in the iron ore. The blast furnace slag that results is used as a base material for the construction of roads and highways. However, the presence of magnesia (MgO) is a critical component in the slag because it lowers the viscosity and improves the efficiency in the removal of impurities from the iron. It also reduces degradation of the refractory lining of the blast furnace and thereby increases its useful lifetime, a significant financial benefit in the production of iron and steel. Dolomite (CaMg[CO3]2) in the limestone provides a source of magnesia when fired and significantly increases the value of limestone for this application. Unfortunately, Mg is a light element and as a result is difficult to quantify or even detect using handheld XRF. Conversely, XRD methods can quite easily distinguish calcite and dolomite as a consequence of their differing crystal structures.
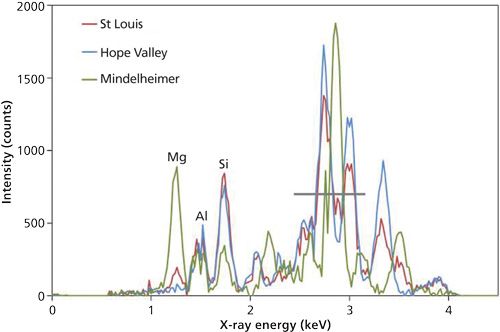
Test results with the handheld XRD prototype for this analysis are shown in Figure 4. It shows three unprepared rock samples corresponding to the samples from St. Louis, Hope Valley, and Mindelheimer Klettersteig (5). Based on this earlier analysis, it was known that the Hope Valley and Mindelheimer Klettersteig rock specimens are dominated by calcite and dolomite, respectively, while the St. Louis sample consists of mainly calcite with minor amounts of dolomite. The signal intensities in Figure 4 are significantly lower than in Figure 3 because the tube excitation voltage is considerably reduced, 4 kV compared to 7 kV for the iron ore application. The part of the spectrum affected by scattering of Rh-L lines is also proportionately greater, but nevertheless the results are consistent with the known compositions. In particular, a dolomite diffraction peak at ~3.5 keV is clearly visible in the St. Louis data but absent in the Hope Valley spectrum, illustrating the detection of dolomite in the former. These measurements could be significantly improved through the use of an anode material in the X-ray tube that has no emission lines in the 2-4 keV range.
Cultural Heritage Applications
The back-reflection EDXRD technique is also likely to find application in the analysis of high value objects because of its completely nondestructive characteristic. The detailed scientific analysis of objects with cultural heritage significance is a growing field. Examples include archaeological artefacts such as pottery (including pigments and glazes), jewelry, any objects made from stone or rock, and art pieces such as paintings and sculptures. Studies of this type are usually carried out either to answer questions relating to origin or manufacture, giving insight into the material history of the objects, or to understand the stability and deterioration of materials to ensure proper conservation and develop new conservation methods. Instrument portability is a great advantage in this application area, enabling the instrument to be taken to the target object, and a handheld XRD device would certainly find application in museums and galleries.
As previously mentioned, a handheld instrument based on the back-reflection EDXRD technique cannot compete with the performance of laboratory diffractometers. However, this drawback is a consequence of the achievable resolution of energy-resolving detectors which, in this context, is quite limited even for state-of-the-art detectors such as silicon-drift devices. Our studies have recently demonstrated the implementation of the back-reflection technique in a high-resolution configuration at the Diamond Light Source synchrotron at Harwell in the United Kingdom (6). The benefit of using a synchrotron is that it generates intense X-ray beams by accelerating a beam of electrons around a circular vacuum tube at approximately the speed of light. As the electrons circle the tube, they emit energy with wavelengths in the range of 0.1-100 nm. However, to produce X-rays with enough intensity to generate high analyte signals with low background noise, the circumference of the vacuum tubes are in the order of several hundred meters. These are such large, specialized facilities that only about 40 synchrotron light sources exist throughout the world. Naturally, this analysis method is restricted to objects that can be taken to the synchrotron. Although acquisition times would be considerably longer, a laboratory version of the high-resolution configuration is certainly possible and constitutes a direction of future research for our laboratory.
Mineral or Phase-Specific XRD Technology
The second novel XRD technique under development by our laboratory enables the enhancement of the diffraction signal of a specific mineral or phase within a mixture. Mineral or phase-specific XRD is suited to applications in which the user is interested in one key mineral or phase, such as an important impurity in the sample. The presence or absence (within the limit of detection) of a specific phase can be determined with this technique.
Phase specificity in the new XRD technique relies on finding coincidences in the ratios of crystal d-spacings and elemental characteristic X-ray energies. Such coincidences can be exploited so that the two crystal planes diffract through the same scattering angle at two different X-ray energies, as illustrated in Figure 5. An energy-resolving detector placed at the appropriate scattering angle will detect a significantly enhanced signal at these energies if the target mineral or phase is present in the sample.
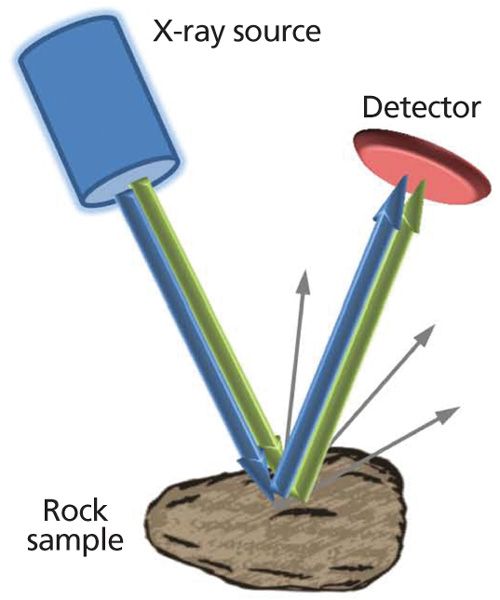
Mathematically, the basis of the technique can be explained as follows. The well-known Bragg law for diffraction of X-rays from crystal planes can be expressed in the energy domain as follows:
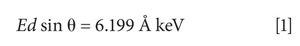
where E is the photon energy, d is the crystal d-spacing, and θ is the scattering angle. For the coincidence-based XRD method to work, the following equation must be satisfied:
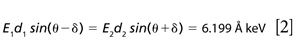
where δ must be small (the coincidence criterion), E1 and E2 are characteristic energies emitted simultaneously by the X-ray tube, and d1 and d2 are d-spacings of the target mineral. Rearranging gives
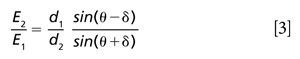
showing that the ratio of characteristic energies must closely match the inverse ratio of d-spacings.
For any given target mineral or phase the anode material must be carefully chosen to meet the coincidence criterion. As an example, quartz can be selectively detected by exploiting the following coincidence: quartz d-spacings of 2.2366 and 2.1277 Å (ratio 1.0512) and Pd (palladium) characteristic emission lines Lα1 at 2.838 keV and Lβ1 at 2.990 keV (ratio 1.0536), for which 2θ = 154.6° and δ = 0.3°. The specific detection of quartz in an unprepared mudstone (which is a type of fine grained sedimentary rock whose original constituents were clays or muds), known to contain quartz, is illustrated in Figure 6.
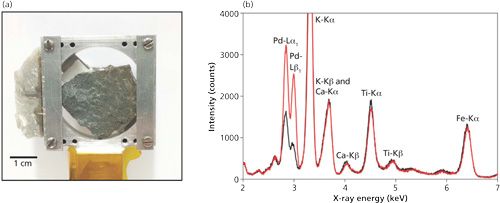
Figure 6: (a) A mounted mudstone rock sample known to contain quartz. (b) The combined EDXRD and EDXRF spectra for the on- (red) and off-coincidence (black) measurements, illustrating the signal enhancement because of quartz near 3 keV. The more prominent XRF peaks are labeled.
In general, for any given target phase several candidate characteristic X-ray energy and d-spacing ratios can be found. Consequently, one of the parameters that can be chosen is the scattering angle. A wide range of angles can, in principle, be considered, but there are several advantages to choosing a high scattering angle, for example 2θ > 150°. A key advantage is reduced sensitivity to sample morphology and relaxed constraints on precise positioning of the sample. For example, moving the sample 2 mm further away from the instrument will have a negligible effect on the measurement and sample morphology on a similar scale is also tolerable. This benefit works in much the same way as in back-reflection EDXRD, though the mineral-specific XRD technique does not have the extreme insensitivity engendered by the former. Fortuitously, it also turns out that a high scattering angle improves specificity and signal intensity, and reduces sensitivity to poor powder averaging. Just as for handheld XRD based on back-reflection EDXRD, a high scattering angle additionally favors a compact instrument design.
Retained Austenite in Steel
An exciting potential application of this technology is the quantification of retained austenite in steels using a handheld or production line instrument. Austenite is a high-temperature allotrope of iron or iron alloy that allows higher levels of carbon to be dissolved in the iron at elevated temperature. As the steel cools, the crystal structure changes and other allotropes (phases) form, including ferrite and martensite. The amount of austenite retained in the microstructure is an important factor in determining some of the strength characteristics of the steel. Depending on the intended use of any given steel, the retained austenite may be detrimental to the mechanical properties (7). For example, the tool and die industry requires minimum amounts of retained austenite and higher levels of ferrite and martensite because maximum hardness and resistance to wear is usually crucial in these applications and austenite reduces the attainable hardness. Conversely, some retained austenite is beneficial in the bearing and gear industry because it suppresses crack propagation. During the manufacture of certain steels the amount of retained austenite must be tightly constrained through optimization of the heat treatment of the steel.
Key to this control is accurate measurement of the retained austenite in the steel as it comes off the production line. Although austenite in steels has traditionally been carried out by metallographic equipment, more recently laboratory-based XRD instrumentation has been considered the most accurate method especially at low concentrations below ~15%. However, the main disadvantages of using XRD for this application is that it is relatively expensive, and also the samples have to be brought back to the laboratory for analysis. By enhancing the diffraction signal specifically from the austenite phase in a steel sample, phase-specific XRD can achieve relatively high sensitivity to low concentrations of this critical phase. It is easy to envisage a handheld “retained austenite sorter” that could be used to quickly accept or reject finished parts based on a threshold amount of retained austenite, analogous to the use of handheld XRF devices in scrap metal sorting. A handheld instrument would cost a fraction of the price of laboratory diffractometers and could prove very desirable for this type of application.
Analysis of Quartz in Mining Samples
Another possible application of the mineral-specific XRD technique is the detection and quantification of crystalline silica (quartz) in mine dust, which has been collected on respiration filters of mine workers. Crystalline silica is responsible for silicosis (8), so there are strict protocols in place for the measurement and control of exposure of mine workers to airborne crystalline silica. Currently there are over 2000 service laboratories worldwide that are carrying out this type of analysis. This application is an excellent example of the required detection of a specific mineral within a potentially complex mixture. As in the retained austenite application, XRD methods are considered to be the most accurate and also the most widely applicable to all types of mine dust, compared to other competing methods such as infrared spectroscopy.
The specific detection and quantification of quartz also has potential in other market segments. For example, quartz is a common impurity in iron ores and, because it is a very hard mineral, its presence influences decisions on the ore processing methods. Rapid quantification of the quartz in these ores, perhaps alongside an analysis of the iron mineral content by a back-reflection XRD instrument, would allow rapid decision-making at the mine site on blending different grades before initial processing of the iron ore.
Conclusion
This column installment reports significant progress in handheld XRD technology, with the recent development of two enabling techniques. The first technique has been developed as a handheld mineral analyzer, which is intended for the rapid mineral identification and quantification of ore-based mining samples in the field through a combination of X-ray diffraction and X-ray fluorescence. This technique will be capable of directly analyzing the mineralogical components of a sample within a few minutes with no sample preparation--traditionally beyond the capabilities of conventional XRD equipment.
The second development is based on a phase-specific XRD technique, which enhances the diffraction signal of a specific mineral or phase within a mixture and is suited to applications in which the user is especially interested in one specific component or impurity in the bulk sample. Both techniques offer the capability of analyzing samples in the field, without having to bring them back to the laboratory for analysis.
Acknowledgments
Graeme Hansford would like to thank Alexander Seyfarth and Terry Smith of Bruker Elemental for their support of these projects and the provision of equipment, the UK’s Science and Technology Facilities Council for funding support.
References
(1) G.M. Hansford, J. Appl. Crystallogr.44, 514-525 (2011).
(2) G.M. Hansford, Nucl. Instrum. Methods Phys. Res., Sect.A728, 102-106 (2013).
(3) A. Seyfarth and B. Kaiser, “History of Handheld XRF,” FIRST Newsletter, Issue 23 (2013), Bruker Elemental.
(4) University of Leicester Press Release: http://www2.le.ac.uk/offices/press/press-releases/2014/january/innovative-handheld-mineral-analyser-2013-2018the-first-of-its-kind2019.
(5) G.M. Hansford, S.M.R. Turner, D. Staab, and D. Vernon, J. Appl. Crystallogr.47, 1708-1715 (2014).
(6) G.M. Hansford, S. Turner, N. Karim, and J. Hall, “Completely Non-Destructive High-Resolution XRD Analysis of Cultural Heritage Objects,” presented at the 64th Denver X-ray Conference, Westminster, Colorado, 2015.
(7) D.H. Herring, “A Discussion of Retained Austenite,” IndustrialHeating.com, March 2015. Available at http://www.heat-treat-doctor.com/documents/RetainedAustenite.pdf.
(8) S. Laney et al., Occup. Environ. Med.67, 652-656 (2010).

Robert Thomas has more than 40 years of experience in trace-element analysis. He is the principal of his own freelance writing and scientific consulting company, Scientific Solutions, based in Gaithersburg, Maryland.

Graeme Hansford received his PhD in high-resolution infrared spectroscopy at the University of Cambridge in the UK and worked for 10 years developing balloon- and aircraft-born instruments for atmospheric measurements. In 2006, he moved to the Space Research Centre at the University of Leicester’s Department of Physics and Astronomy, to develop X-ray instrumentation for space missions. Since 2010, his work has focused on novel XRD techniques, instrumentation, and applications. Please direct correspondence to: gmh14@leicester.ac.uk.

Applications of Micro X-Ray Fluorescence Spectroscopy in Food and Agricultural Products
January 25th 2025In recent years, advances in X-ray optics and detectors have enabled the commercialization of laboratory μXRF spectrometers with spot sizes of ~3 to 30 μm that are suitable for routine imaging of element localization, which was previously only available with scanning electron microscopy (SEM-EDS). This new technique opens a variety of new μXRF applications in the food and agricultural sciences, which have the potential to provide researchers with valuable data that can enhance food safety, improve product consistency, and refine our understanding of the mechanisms of elemental uptake and homeostasis in agricultural crops. This month’s column takes a more detailed look at some of those application areas.
Portable and Wearable Spectrometers in Our Future
December 3rd 2024The following is a summary of selected articles published recently in Spectroscopy on the subject of handheld, portable, and wearable spectrometers representing a variety of analytical techniques and applications. Here we take a closer look at the ever shrinking world of spectroscopy devices and how they are used. As spectrometers progress from bulky lab instruments to compact, portable, and even wearable devices, the future of spectroscopy is transforming dramatically. These advancements enable real-time, on-site analysis across diverse industries, from healthcare to environmental monitoring. This summary article explores cutting-edge developments in miniaturized spectrometers and their expanding range of practical applications.