ICP-MS Analysis of Trace Selenium in the Great Salt Lake
January 2007. Because of concerns over increasing levels of anthropogenic pollutants in the Great Salt Lake in Utah, the Utah Division of Water Quality recently conducted a roundrobin study of selenium in ambient and spiked Great Salt Lake waters to determine the most practical analytical technique for its detection. Of the techniques applied, only hydride generation atomic absorption and octopole reaction system ICP-MS provided acceptable results.

The Great Salt Lake in Utah is one of the Western Hemisphere's most important migratory bird habitats. As such, the water quality of the lake and its tributaries can have far-reaching consequences. However, while there are currently narrative standards for anthropogenic pollutants applied to the Great Salt Lake, actual numeric standards for metals have not yet been developed by the Utah Division of Water Quality. To this end, a recent cooperative effort (GSL Water Quality Steering Committee [1]) including The Nature Conservancy, the Great Salt Lake Alliance, Friends of Great Salt Lake, the State Division of Water Quality, the Jordan Valley Water Conservancy District, and Kennecott Utah Copper is working to recommend numeric water quality standards for some metals in the open waters of the lake (2).
Beginning with selenium, a nationally selected panel of scientific experts will analyze existing data and recommend additional research to determine how much selenium is entering the lake due to human activities, and how it is affecting the Great Salt Lake's bird population. The plan calls for recommendation of numeric water quality standards for selenium in the Great Salt Lake by the Utah Department of Environmental Quality (DEQ) to the EPA by the end of 2007. Critical to determining baseline data for Se in the lake is the validation of a reliable analytical method.
Method Criteria
To meet the needs of the study, the successful analytical method must meet several requirements:
- High sensitivity must be achieved (due to required minimum detectable limit (MDL) of <0.5 ppb in the undiluted sample).
- Analysis must be performed with minimum sample dilution in order to retain sensitivity.
- The method must tolerate high total dissolved salt concentrations with minimum signal suppression and minimum signal drift.
- Method must avoid significant spectral interferences on at least one Se isotope.
- Method must be simple and reliable.
Because of the requirement for the lowest possible detection limit (<0.5 ppb), less sensitive elemental analysis techniques such as inductively coupled plasma-optical emission spectroscopy (ICP-OES) or flame atomic absorption (FAA) were not considered. More sensitive techniques such as hydride generation atomic absorption (HGAA), graphite furnace atomic absorption (GFAA) or ICP-mass spectrometry (ICP-MS) were used. However, the extreme salinity of the Great Salt Lake has rendered these techniques problematic in the past. Great Salt Lake total dissolved solids range from ~9% to ~28%, depending upon season and location (Table I). Furthermore, Great Salt Lake water is alkaline, pH ~ 8.6 on average. At these extreme total dissolved solids values, the Se signal is enhanced greatly using both graphite furnace AA and conventional ICP-MS. Recent analytical results for Se have ranged from about 1 μg/L using hydride AA to about 120 μg/L using graphite furnace AA. Bromide, chloride, and sulfates are the primary sources of these interferences (3). Additionally, the argon dimers (40 Ar40 Ar+ , 40 40Ar38 Ar+ ) can exaggerate the ICP-MS signal when 80 Se or 78 Se is used as the measured isotope (4). Another complicating factor is that the Great Salt Lake is intensely stratified with a sharp chemocline occurring at approximately mid-depth (5 m). The water column at and below this chemocline (the deep brine layer) is anaerobic and presents a highly reducing environment that can cause sample preservation, storage, and preparation difficulties.
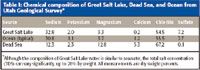
Table I: Chemical composition of Great Salt Lake, Dead Sea, and Ocean from Utah Geological Survey*
Round-Robin Study
As a result of these challenges, a recent round-robin study among seven laboratories was conducted by the Utah Division of Water Quality to compare ambient level Se measurements and low-level spike recoveries of Se in Great Salt Lake waters using multiple techniques. The goal of the study was to determine the most practical analytical method for use in continuing studies. In addition to GFAA and HGAA, ICP-MS was selected for inclusion in the round robin primarily because of its reputation for high sensitivity and potential for rapid multielement analysis without complicated sample preparation procedures. However, ICP-MS also has the reputation for low tolerance to samples containing high dissolved solids that could require unacceptably high dilution factors. It is also known that conventional ICP-MS analysis of selenium can suffer severe interferences in samples containing chlorine or bromine, both of which occur in high concentrations in the Great Salt Lake. For these reasons, conventional (noncell) ICP-MS was not considered optimal for this application. However, collision/reaction cell ICP-MS, known to be effective at reducing polyatomic interferences in many sample types, was considered a possibility. Both conventional ICP-MS and passive and dynamic collision/reaction cell instruments were evaluated and the results compared with those obtained using GFAA and HGAA from multiple laboratories.
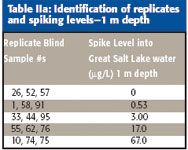
Table IIa: Identification of replicates and spiking levels-1 m depth
Sample Collection and Preservation
Two samples (1-m and 7-m deep) were collected from a single location near the middle of the south arm using a peristaltic pump. Sample water was passed through a 0.45-μm membrane filter and stored in 10-gal carboys. These samples were then acidified with nitric acid to pH < 1 and transported to Environmental Resource Associates (Aurora, Colorado) for preparation of replicates and spiking. In addition to native Great Salt Lake water, triplicate spikes were prepared according to Table II. Sample identification numbers were applied randomly to all sample replicates and distributed "blind" to participating laboratories.
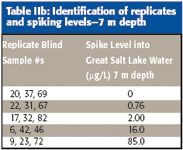
Table IIb: Identification of replicates and spiking levels-7 m depth
A total of 36 samples were submitted to each of seven participating laboratories for analysis. Because the samples were submitted blindly, the analyst did not know which samples were replicates and which were spikes. Each participating laboratory was free to choose the analytical technique, depending upon their proficiency and available instrumentation. In all cases, standard analytical methods were employed. Analytical techniques included HGAA (EPA 270.3) GFAA (EPA 200.12), conventional (noncollision cell) ICP-MS (EPA 200.8) (Agilent 7500a, Agilent Technologies, Palo Alto, California), dynamic reaction cell (DRC) ICP-MS (EPA-200.8 modified) (PerkinElmer Elan DRC II, PerkinElmer, Shelton, Connecticut), and octopole reaction cell ICP-MS (EPA 200.8 modified) (Agilent 7500ce). Although all the techniques employed are commonly used for analysis of selenium in various environmental samples, Great Salt Lake water presented a significant challenge to most. Because of the very high level of dissolved solids, both spectral and nonspectral interferences were evident for all techniques evaluated. The levels of interferences ranged from slight to severe.
The final round-robin results for all seven participating laboratories are presented in Table III. Two techniques, hydride AA and octopole reaction cell ICP-MS, provided sufficiently accurate and precise results. The remaining techniques, GFAA, DRC ICP-MS. and conventional (noncell) ICP-MS, showed significant positive bias and yielded unusable results. GFAA is known to suffer from interferences from chlorides and sulfates, which can be mitigated by the addition of nickel nitrate, but not eliminated. In the case of Great Salt Lake waters, the Cl and SO4 concentrations are too high. Similarly, conventional ICP-MS suffers from too many polyatomic interferences to measure selenium in high Cl, high Br matrices. The DRC ICP-MS situation is a bit different. Like the octopole reaction cell instrument, the DRC ICP-MS system uses a collision/reaction cell to remove polyatomic interferences before they enter the mass spectrometer and are measured. Typically, reactive gases are used with the goal of separating the analyte from the interferent by shifting either the mass of the polyatomic interference or the analyte to a new mass via ion–molecule reaction. In this case, oxygen was used as the reaction gas. Oxygen readily combines with Se+ to form SeO+ , effectively shifting the Se signal away from the Ar2+ interferences at m/z 78 and 80. The resulting analyte signal is measured at m/z 94 (78 Se16 O+ ), and m/z 96 (80 Se16 O+ ). However, the very large positive bias in the results, approximately 500–800%, suggests the presence of large, unknown interferences at the new masses. Furthermore, very specific reaction chemistry, such as that used in both DRC ICP-MS and hydride AA, limits the scope of the analysis to only a few related analytes. Conversely, octopole reaction cell ICP-MS uses simple, universal conditions to remove polyatomic interferences generically while maintaining the analyte (in this case, selenium) at its actual mass. Therefore, the use of octopole reaction cell ICP-MS presents the advantage over hydride AA of being able to simultaneously measure other trace elements in addition to selenium in saline samples. This will prove useful as the characterization of the Great Salt Lake progresses to other analytes.

Table III: Summary results of round-robin study
Overall, octopole reaction cell ICP-MS was chosen for continued work because of its sensitivity, lack of bias, multielement capability, and ease of use.
Octopole Reaction Cell ICP-MS Analysis of Great Salt Lake Waters
In addition to the known limitations to the analysis of Se by ICP-MS in highly saline waters that were outlined previously, other challenges to accurate selenium measurement by ICP-MS include:
- Selenium occurs in several isotopic forms — reducing the response of any one individual isotope (Table IV).
- Selenium has a high ionization potential, making it difficult to ionize in argon plasma, particularly in the presence of easily ionized elements like sodium.
- There are many common polyatomic interferences affecting all selenium isotopes (Table IV).
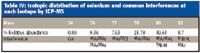
Table IV: Isotopic distribution of selenium and common interferences at each isotope by ICP-MS
These limitations are exacerbated in highly saline samples such as Great Salt Lake water because the major components are easily ionizable cations, Na and K, plus Cl and some Br. Easily ionized elements suppress the ionization of high ionization potential elements such as Se by preferentially ionizing in the plasma. Chlorine forms ArCl+ in the plasma, directly overlapping 77 Se, the most commonly measured isotope. Bromine forms BrH+ , which interferes with 80 Se and 82 Se, and finally Ar2+ produced in the plasma overlaps 76 Se, 78 Se, and 80 Se. Although it is possible to compensate for some of these interferences through the use of mathematical correction equations, it is not possible to correct for the interferences at isotopes 76, 78, 80, and 82. This leaves only 77 as a useful isotope, and the high and variable chloride concentration of Great Salt Lake samples makes this unreliable. As a result, collision/reaction cell ICP-MS was considered.
Collision/Reaction Cell ICP-MS
Collision/reaction cell ICP-MS is a technique that uses ion-molecule chemistry to eliminate polyatomic interferences from the mass spectrum of an ICP-MS. The mechanisms are varied and are described elsewhere (5), but the technique has enabled ICP-MS to become virtually free of polyatomic interferences. One type of collision/reaction cell uses an octopole ion guide within a small collision cell, called an octopole reaction system (ORS, Agilent Technologies) to achieve the desired reduction in polyatomic interferences. In this work, the instrument was operated with the ORS in reaction mode using pure H2 cell gas, which effectively eliminates plasma-based spectral interferences (including Ar2+ ) from the mass spectrum. The result is that the two most abundant isotopes of selenium, 78 and 80, are made available for interference-free analysis. As shown in the following equation, the mechanism of removal of the Ar2+ interference is by simple proton transfer:
Ar2+ + H2 → Ar2H+ + H
Se reacts very slowly with H2, but the Ar2+ interference is rapidly moved one mass higher (Ar2H+ ) and away from Se. In addition, because hydrogen is the lightest cell gas, loss of analyte signal due to scattering in the reaction cell is minimized. Because the Ar2+ interference is independent of the sample matrix (it is a product of the argon in the plasma only), interference removal is independent of the sample composition or concentration. This is important because the salt concentration of Great Salt Lake waters can vary considerably. However, if Br is present, it can form BrH+ in the plasma and in the reaction cell as a result of reaction with H2. 79 BrH+ would interfere with 80 Se. Therefore, in this work, 78 Se, the second most abundant Se isotope, was chosen for analysis.

Table V: ICP-MS parameters used
ORS ICP-MS Optimization
The ICP-MS system mentioned earlier was operated in hydrogen mode in order to effectively remove argon-based polyatomic interferences on 78 Se. ICP-MS parameters are presented in Table V. Optimization of cell parameters to reduce Se background at mass 78 involves setting generic conditions including 2 V of energy discrimination and 4 mL/min of hydrogen flow. These conditions work equally well for any argon-based polyatomic and generally are used without further optimization.

Table VI: Analytical sequence including calibrations, blanks, samples, and continuing calibration verification (CCV) standards. In all, 65 analyses were performed in the sequence, including four sets of CCVs and blanks
Determining Sensitivity
The typical response for 78 Se on the ORS ICP-MS system in H2 reaction mode is ~ 1500 cps/μg L-1 . This allows linear calibrations from 0.1 to 1 ppb and a calculated detection limit of less than 10 ppt with a background equivalent concentration (BEC) of 6.45 ppt (Figure 1).
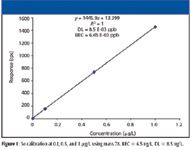
Figure 1: Se calibration at 0.1, 0.5, and 1 μg/L using mass 78. BEC = 6.5 ng/L, DL = 8.5 ng/L.
Dilution and Sample Preparation for ORS ICP-MS Analysis
Preserved Great Salt Lake samples were diluted 50-fold (200 μL into 10 mL) with a diluent containing 0.1% nitric acid and 1% methanol. This reduced the total dissolved solids from ~ 25% to ~ 0.5%. At this dilution factor, a 10-ppt detection limit is required to achieve the desired detection limit of 0.5 μg/L in the undiluted samples.
Standards were matrix matched to samples (0.5% sodium chloride) to compensate for ionization suppression effects, and 5 μg/L germanium was added as an internal standard. Methanol (1%) was added to all standards, samples, and blanks because it is known to enhance the sensitivity for selenium (7). Figure 2 shows the effects of methanol addition to matrix-matched standards. The lower calibration (Figure 2b) was performed without methanol addition; the upper calibration (Figure 2a) with addition of 1% methanol. The response using methanol is approximately three times higher, resulting in improved counting statistics at very low concentrations.
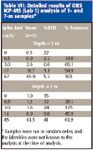
Table VII: Detailed results of ORS ICP-MS (Lab 1) analysis of 1- and 7-m samples*
The samples were then loaded into the autosampler as part of the sequence shown in Table VI, which also included initial calibration standards and blanks and periodic continuing calibration verification (CCV) standards.

Figure 2: Effects of methanol addition on selenium response: (a) 1% methanol added and (b) no methanol added.
Results and Discussion
Results for both the 1-m and 7-m samples as analyzed using the ORS ICP-MS system are presented in Table VII. In general, the data for the 1-m samples are much better in terms of both recovery and precision than that at 7 m. This is attributed to the difference in redox potential between the two sampling depths (that is, the 7-m depth is persistently anoxic). Several factors might be involved. For example, spiking solutions were added as elemental selenium dissolved in 1% nitric acid. It is possible that this Se underwent reduction and precipitation. It is also possible that organic volatile forms of Se could be formed (such as dimethyl selenide) and lost from solution. Further investigation into sample collection, preservation, and preparation is expected to resolve this problem.
As an indicator of both the degree of ionization suppression in the samples compared to the standards and blanks and of the long-term stability of the analysis, internal standard response was monitored over the course of the sequence (Figure 3). If instrument performance had changed as a result of salt buildup in the interface cones or ion optics, it would be apparent from the internal standard recoveries. No net change in instrument response was evident. A single analysis showed significant reduction in internal standard response but did not cause an outlier in analyte response. The cause of this anomaly is unknown. In addition to raw sensitivity (response), calibration accuracy also was monitored periodically by checking 50-ppt CCV samples and blanks after every 10 sample runs. The results are shown in Table VIII.

Figure 3: Internal standard (germanium) response over the course of the analytical sequence including both 1- and 7-m samples.
Conclusions
Because of the high levels of dissolved salts in Great Salt Lake water, most conventional techniques for the analysis of selenium in waters are inappropriate, resulting in significant positive bias. Of the techniques evaluated, including GFAA, conventional ICP-MS (no collision/reaction cell), DRC ICP-MS, hydride AA, and ORS ICP-MS, only hydride AA and ORS ICP-MS were able to provide consistent and presumably accurate measurement of selenium from ambient levels to samples spiked as high as 85 ppb. Because there are no suitable certified reference materials available (appropriate concentrations in a similar matrix), accuracy was determined from spike recoveries. ORS ICP-MS provides the added benefits of simple sample preparation, high sample throughput, and capability for simultaneous multielement analysis. As such, we (Utah Division of Water Quality) have recommended that EPA begin the process of certifying ORS ICP-MS as an available method for selenium analysis in hypersaline samples such as the Great Salt Lake.
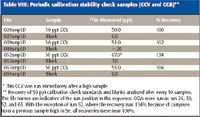
Table VIII: Periodic calibration stability check samples (CCV and CCB)**
References
(1) http://www.deq.utah.gov/issues/GSL_WQSC
(2) http://nature.org/wherewework/northamerica/states/utah/science/art14352.html
(3) EPA Methods 200.12 and 270.2, www.nemi.gov
(4) D.W. Koppenaal, D.G. Eiden, and C. Barinaga, J. Anal. At. Spectrom. 19, 561–570 (2004).
(5) http://geology.utah.gov/online_html/pi/pi-39/pi39pg9.htm
(6) S.D. Tanner, V.I. Baranov, and D.R. Bandura, Spectrochim. Acta, Part B57, 1361–1452 (2002).
(7) E. Larsen and S. Stürup, J. Anal. At. Spectrom. 9, 1099–1105 (1994).
William O. Moellmer and Theron G. Miller are with the Utah Division of Water Quality.
Steve Wilbur and Emmett Soffey are with Agilent Technologies, Inc.
High-Speed Laser MS for Precise, Prep-Free Environmental Particle Tracking
April 21st 2025Scientists at Oak Ridge National Laboratory have demonstrated that a fast, laser-based mass spectrometry method—LA-ICP-TOF-MS—can accurately detect and identify airborne environmental particles, including toxic metal particles like ruthenium, without the need for complex sample preparation. The work offers a breakthrough in rapid, high-resolution analysis of environmental pollutants.
Trending on Spectroscopy: The Top Content of 2024
December 30th 2024In 2024, we launched multiple content series, covered major conferences, presented two awards, and continued our monthly Analytically Speaking episodes. Below, you'll find a selection of the most popular content from Spectroscopy over the past year.
